Observations on British Issus (Hemiptera, Issidae) with reference to development, periodicity and ecology
P.F. Whitehead & R.S. Key
Introduction
Two species of Issus occur in Britain, although in 1960 Le Quesne knew only one. Issus coleoptratus (F., 1781) is widespread in southern England (Map 1), occurring also in Wales and Ireland. Issus muscaeformis (Schrank, 1781) is a relatively rare species of western Britain, accorded Notable status by Kirby (1992). Although some may be widespread, all Issus species are regarded as flightless and thus of some 'conservation' significance, especially those found on archipelagos (Remane, 1985; Quartau, 2008). The discovery of a distinctive nymph at Usk, Monmouthshire, during 2006 (Fig. 6), brought into focus how little is known of their immature stages and prompted this paper, which has been written by P.F.W. and illustrated by R.S.K using Helicon autofocus software.
Identification of adult Issus coleoptratus and I. muscaeformis
Issus coleoptratus is distinct amongst European Issus in that the external longitudinal forewing veins are effaced distally in the female, but are prominent throughout in the male (Holzinger, Kammerlander & Nickel, 2003, fig. 243a, b). There is no forewing sexual dimorphism in I. muscaeformis. The forewings of I. coleoptratus are frequently largely concolorous often with a greenish tinge. Although there may be some evidence of forewing banding (Dolling, 1982, unpublished), it is never as well-marked as in the more strongly pigmented I. muscaeformis in which the forewing variegation may be accentuated (Fig. 1) by pale hyaline areas (Holzinger, Kammerlander & Nickel, 2003, plate 40, fig. 4b; Thomas, 2006). I. coleoptratus usually has a discal spot on the forewings (Dolling, 1991, fig. 75; Holzinger, Kammerlander & Nickel, 2003, plate 40, figs 3a, 3b). In I. muscaeformis the forewing cross-veins are simpler and less branched (Fig. 1) than those of I. coleoptratus which frequently divaricate (Dolling, 1991).
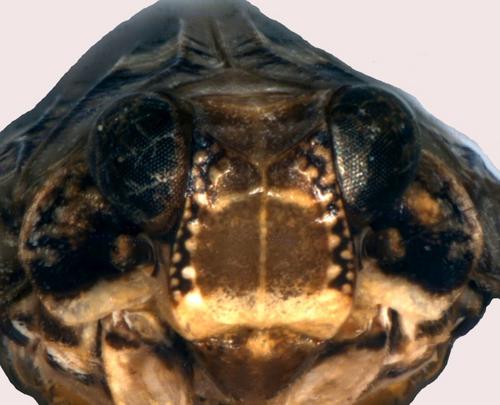
The frons patterns of both species are distinct (Figs 2, 3). The frons of I. coloeoptratus has a contrasting heavily pigmented dark zone just below the vertex (Fig. 2) which is absent in I. muscaeformis. In broad terms, the colour patterns of the frons of adults are a developed reflection of those of their nymphs (Figs 5, 6). Adult I. muscaeformis show a clear pale or white horizontal band just above the epistomal suture which runs from side to side without interruption (Fig. 3; Holzinger, Kammerlander & Nickel, 2003, plate 40, fig. 4a; Biedermann & Neidringhaus, 2009 p. 164). This pale band is usually clearly demarcated above and below but on occasion those edges may be somewhat diffusely demarcated (Dr P. Kirby, in litt.,13.xi.2006). Although the lower frons is pale in I. coleoptratus, no such strongly-defined cross-band is present (Fig. 2). Consideration of all of these points of distinction will enable adults of both species to be distinguished in the field and remove any doubts about the specific distinctness of the two species (Kirby, 1992). We have only examined female adult I. muscaeformis. The structure of the abdominal terminalia and its sixth sternite are quite distinct in female I. muscaeformis and female I. coleoptratus (Holzinger, Kammerlander & Nickel, 2003 figs 243 and 245).
[In southern Europe a further species, Issus lauri Ahrens, 1814, occurs in thermophilous scrub woodland, open xerothermic sclerophyllous oak woodland and boken macquis on a range of trees. Adults frequently have pale green forewings but forms with almost blackened forewings have been seen on Kefallinia in Greece (P.F.W., pers. obs.). In this species the frons also has a pale transverse band but it lies above a narrower dark band which itself touches the epistomal suture. Unlike I. muscaeformis the frons of I. lauri is markedly darkened from the top down, on occasion by as much as 30% of its length.]
Identification of nymphal Issus coleoptratus and I. muscaeformis
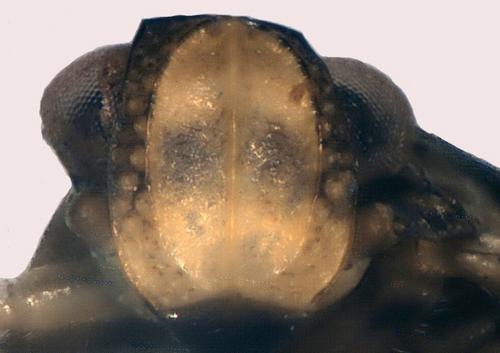
During studies on I. coleoptratus in midland England P.F.W. observed several breeding populations. Some L5 instar nymphs were raised to maturity, and some familiarity with nymphs has been gained. On 12.x.2006 P.F.W. swept an L3 instar Issus nymph at Springdale Farm, Usk, Monmouthshire (VC35 SO48 125m O.D.) on bracken under oak (Quercus) at the edge of a block of ancient oak woodland. It is highly variegated with broadly annulated leg members (Fig. 4) and its identification initially proved problematical. Mr M. Webb of the Department of Entomology, British Museum.Natural History, London, kindly made available examples of L4 stage I. muscaeformis from Wray Castle in the vice-county of Westmorland and North Lancashire (VC69 NY30), collected by W. E. China on 10.x.1945 with adults. The nymphs agreed well in all respects with the Usk L3 nymph. The frons in nymphal I. muscaeformis seen has a single dark horizontal band occupying a medial position (Fig. 6), obvious in the Wray Castle specimens. The frons of nymphal I. coleoptratus have a pale horizontal medial band and more obviously defined sequential banding (Fig. 5), the darker area below the vertex being largely absent in I. muscaeformis.
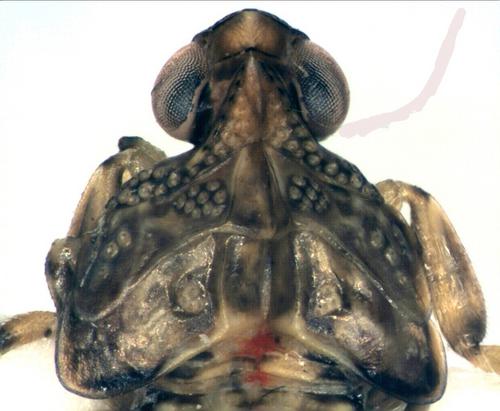
Further distinctions, which we take to be specific, can be found in the arrangement of sensory pits on the wing pads. As the nymphal instars progress, their forewing pads extend downwards at the sides (shown also in Zenner, Stöckmann & Niedringhaus, 2005), until by the final L5 instar they acquire a rudimentary venation (Fig. 8). A group of sensory pits is situated at the top of each of the forewing pads near their inner angles (Figs 4, 7, 8). In nymphal I. coleoptratus (certainly in L2-L5 instars) these are large, clearly annulated and typically number ten; they are somewhat dispersed and are only occasionally contiguous. In nymphal I. muscaeformis seen these pits are smaller, less broadly annulated, typically number seven and tend to be contiguous. For these reasons we recognise the Usk nymph as I. muscaeformis. The variegated, broadly and clearly darkly annulated leg members are also believed to be characteristic of I. muscaeformis at all growth stages (Thomas, 2006). Although nymphal I. coleoptratus are generally less strongly pigmented in this regard, there should be some awareness of occasional more heavily pigmented I. coleoptratus which some recorders have mentioned to us.
Morphometry of nymphal Issus coleoptratus and I. muscaeformis
We record the overall body lengths of dry nymphal instars of British Issus species as follows: L2, 1.9 mm; L3, 2.5 mm; L4, 2.4 - 2.8 mm; L5, 3.6 mm. These figures based on a small sample include only three nymphal I. muscaeformis (L3, L4) and should be regarded as generalised and merely indicative.
Periodicity of nymphal Issus coleoptratus and I. muscaeformis
Dolling (1982, unpublished) and Kirby (1992) outlined problems surrounding the periodicity of Issus, implying that it could be either annual or biennial, or even 'disorganised' i.e. not conforming to recognisable definable time-spans. The finding of L2 and L4 I. coleoptratus nymphs together at Malvern, Worcestershire on 28.x.2006 implies that periodicity and/or nymphal longevity may be variable at a single location. Ameliorated small-scale microclimates or thermal hot-spots in the crowns of woodland edge trees may facilitate this.
We have records of known instar Issus nymphs, all but three of which are I. coleoptratus, during the following months: L2: September, October; L3: October; L4: February, April, May, October; L5: May, August, October, with adults from May to September and in November (an overwintering adult is still awaited). Early oviposition in June could produce L3 stage nymphs by October and late oviposition could explain an L2 stage nymph in October; it may be that the phased attainment of maturity for this flightless group is a ploy to maximise survival over time in line with resource availability dynamics, e.g. at the range edge. We record newly moulted adults at the end of May and teneral adults in June; a captive L5 stage nymph became very sluggish and immobile a few days prior to its final moult on 29.v.2009.
The L2 and L4 instar nymphs together on ivy at Malvern on 28.x.2006 highlight this problem of periodicity, and suggest that all but the first nymphal stage overwinter, sometimes synchronously. To determine the periodicity of the instars it would be necessary to establish their longevity which is unknown, but if the longevity of the L2 nymphal instar was to be extended, as seems likely to us, then nymphs would certainly overwinter at least twice, ongoing development being governed by seasonality, or perhaps more importantly, lack of it. It is thus tempting to suppose that, at least at 52°N in Britain, periodicity is indeed 'disorganised' (sensu Kirby, 1992), or more exactly perhaps, randomised. It is possible that prevailing conditions i.e. of locally ameliorated or man-modified climates determine whether nymphs overwinter at instar L4 or L5. The discovery of adult I. muscaeformis in Merionethshire in late June by R.S.K. and in North Lancashire and Westmorland by Mr A.P. Foster in early July provides an indication of comparable periodicity in both species. The existence of L4 instar nymphal I. coleoptratus in October removes the doubts expressed by Dolling (1991) about the distinct or different periodicity of the two species. It seems probable that adults of both species may at least attempt to overwinter, Issus sp., perhaps I.. muscaeformis having been found as late as 11.xi.1998 on Yew (Taxus baccata L.) at Blaise Castle Estate, near Bristol (VC34 Alexander, in litt., 26.i.2010) and 2.xi.2006 also on Yew (Taxus baccata L.) at Gait Barrows (Thomas, 2006). The ability of nymphs and possibly adults to overwinter successfully in Britain is a determinant of the distribution of both species.
Distribution and ecology of Issus coleoptratus and I. muscaeformis
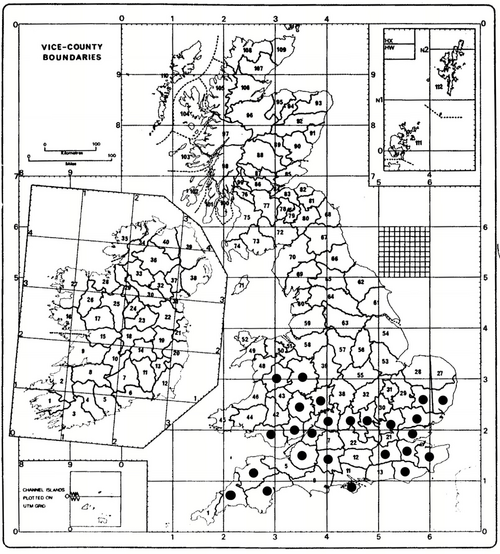
Issus coleoptratus is widespread in the Palaearctic Region and the late Professor R. Remane (in litt.,10.vii.2008) recorded it as far south as Algeria and Tunisia. It is widespread in southern and midland England (Alexander, in litt., 26.i.2010; Dolling, 1982, unpublished; Whitehead, pers. obs) and is more eurytopic than I. muscaeformis. It occurs in many English vice-counties (2, 3, 4, 6, 8, 10, 14, 15, 16, 17, 18, 19, 20, 23, 24, 25, 26, 33, 34, 35, 36, 37, 40, 41 and 47) indicating a wide geographical spread across southern England (Map 1). The Irish status is unclear to us but there is a breeding record from Torc Woods, Killarney, County Kerry, on 6.vi.1994 (Alexander, in litt., 26.i.2010) and it is cited as Irish by Dolling (1982, unpublished).
It is sometimes stated that ivy Hedera is a shelter-plant for British Issus but we confirm breeding on ivy based on numerous repeated observations of early instars and adults on that plant. Summer adult I. coleoptratus s on ivy (Hedera helix L.) at Malvern, Worcestershire often appear noticeably green following prolonged feeding on this plant. On Bredon Hill, Worcestershire, adults have been found on ivy in old Blackthorn (Prunus spinosa L.) hedges in summer and at beech and oak woodland edges at Brown's Folly, Bathampton, Somerset. Fidelity to ivy is confirmed by Alexander (in litt., 26.i.2010) who observed I. coleoptratus nymphs on ivy at Scotney Castle Park, Kent (6.v.1987) and adults on ivy in August (17.viii.1982), September (4.ix.1986) and October (21.x.2005) at Littleton Down, Ventnor, Isle of Wight, Blakes Wood, Essex and Exeter, Devon respectively. Of interest is a further record of I. coleoptratus on cliff-draped ivy at Branscombe Mouth, Devon on 22.vi.2003 which mirrors the habitat of I. muscaeformis on the walls of Wray Castle. In North Essex an adult and nymph were observed on ivy at Colchester on 17.v.2002, and an adult at Thorpe-le-Soken under an ivy-covered oak tree in a garden on 10.vii.2003 (J. Bowdrey, in litt., 29.i.2010). At Gourdon, in Les Alpes Maritimes, Provence (775 m altitude), an adult female I. coleoptratus was on ivy (Hedera helix L.) on oak on crags above the Gorge du Loup on 18.vi.2008 (P.F.W., pers. obs.).
This association with ivy seems to be an important one for many species of Issus. The late Professor R. Remane examined no fewer than 2000 specimens of Issus from Macaronesia (Remane, 1985) describing numerous species. Some were found on herbs e.g. on Euphorbia spp., some on pine Pinus, but most were inhabitants of Neogene Laurel forests, were they were held to be polyphagous on sclerophyllous and other trees. On La Gomera, P.F.W. found Issus sp. breeding in numbers on ivy (Hedera canariensis Willdenow) suspended from overhanging trees at Chipperon de Epina on 28.iv.2008, a host plant which Remane seems to have missed. The area is largely thermophilous, open, Fayal-Brezal woodland (Fernández, 2002) on the eastern boundary of the National Park. The specimens are represented only by later instar nymphs so cannot yet be determined.
Issus coleoptratus has been recorded (Holzinger, Kammerlander & Nickel, 2003; Nickel, 1997) on Hazel (Corylus avellana L.), birch (Betula), lime (Tilia), elm (Ulmus), oak (Quercus), Sorbus and maple (Acer). British experience reflects this wide woody plant utilisation. Alexander (in litt., 26.i.2010) recorded nymphs on Hazel at Brockhampton Park, Herefordshire on 5.vi.1984, an adult on birch at Foxes Bridge Marsh, Gloucestershire on 10.viii.2002 and a nymph on Wild Service Sorbus torminalis (L.) Crantz at Rudge Wood, Fownhope, Herefordshire on 27.v.1984. At Stour Wood, Wrabness, North Essex, adult females were on Silver Birch Betula pendula Roth on 24.x.1996 and on oak Q. robur L. on 19.xi.2000 (J. Bowdrey, in litt., 29.i.2010). At Weydown Common, Surrey, on 1.vii.1983 Alexander observed a nymph on holly Ilex aquifolium L., apparently a rare host plant cited by Le Quesne (1960).
Issus coleoptratus is markedly synanthropic (P.F.W., pers. obs.) occurring in rural, suburban and urban gardens on such shrubs as Mock Orange (Philadelphus coronarius L.) and Lilac (Syringa vulgaris L.). At Evesham, Worcestershire, L4 instar nymphs occurred on Clematis armandii Franch. in May and on ivy Hedera 'Cavendishii' also in May, where an especially favoured plant is the large-leaved ivy Hedera colchica Koch (P.F.W., pers. obs.). In Worcester city I. coleoptratus is associated with leafy Georgian town squares where L5 instar nymphs have been found on evergreen sclerophyllous Garrya elliptica Dougl. ex Lindl. iIn summer. An affinity for evergreen garden plants with firm leaves is therefore demonstrated, and it is believed that I. coleoptratus breed on these, some garden populations showing high fidelity to ivy, but we have no records of precise feeding methods. Nymphs of I. coleoptratus, and presumably also those of I. muscaeformis, are capable of jumping distances ranging from 240 mm to 350 mm when threatened (P.F.W., pers. obs.). At Malvern, Worcestershire, an association between I. coleoptratus and the rare dryinid Dryinus collaris (L. 1767) has been suggested (Whitehead, 2010).
Issus muscaeformis was recorded new to Britain in 1977 (Payne, 1979) from Gait Barrows, mid-Lancashire, followed three years later by a specimen from Eaves Wood, Silverdale (Alexander, 1981). Subsequently, British specimens of I. muscaeformis collected by W.E. China in 1945 from Wray Castle (vide supra) were found in the collections of B.M.N.H., London (Dolling, 1982). The hinterland of Morecambe Bay around Arnside and Silverdale remains a locus classicus with records from mid-Lancashire (VC60), Westmorland and North Lancashire (VC69) and Cumberland (VC70) (Hewitt, in litt., 24.i.2010; Thomas, 2006). To its vice-county distribution we now add Merionethshire (VC48 SH64 Hafod Garregog, 24.vi.1998, fig. 1), Monmouthshire (VC35 SO49 Usk, 12.x.2006, L4 instar nymph, figs 4, 6), and North Cornwall (VC2 SS21) where, as elsewhere in Europe, it is an inhabitant of mixed oak woodland, often of the woodland fringe. Hafod Garregog lies within a National Nature Reserve renowned for its invertebrate interest (Fowles, 1994) and Fig. 1 illustrates the first Welsh specimen of Issus muscaeformis, the Usk nymph being the second. The hitherto unpublished Cornish record is of an adult female found at Stowe Woods by Dr P. Kirby on 11.ix.1985. Oak (Quercus) is regarded as a host plant by Ossiannilsson (1978) and a specimen collected by Mr A. P. Foster from Waterslack Wood (VC69) (not strictly Eaves Wood as has previously been stated), Silverdale on 9.vii.1996 was beaten from oak (Foster, in litt., 21.i.2010; Kirby in litt., 13.xi.2006). The Monmouthshire I. muscaeformis nymph cited here was also associated with oak woodland and other Welsh records, especially in coastal vice-counties, are anticipated. Recent finds from VC69 and VC70 (Thomas, 2006) have been on Sycamore (Acer pseudoplatanus L.) in October 2006 and ash (Fraxinus excelsior L.) in May 2007. Holzinger, Kammerlander & Nickel (2003) add Hornbeam (Carpinus betulus L.) and Hazel (Corylus avellana L.) as potential host plants. There is presently no convincing evidence of synanthropy, but the population on the wall-draped ivy of Wray Castle approaches it, and provides the closest links with I. coleoptratus habitat in Britain.
Occasional records from Yew (Taxus baccata L.) are of particular interest as there is as yet no well-established association between I. coleoptratus and Yew; any Issus nymphs recorded on Yew in the future should be determined. The only record of an Issus nymph on Yew in Britain known to us is that from Lady Park Wood, Monmouthshire (VC35 SO51) on 1.vii.1984, but the specimen was not retained (Alexander, in litt., 29.i.2010). Alexander (vide supra) also recorded an adult Issus sp. on Yew in the limestone gorge area of Blaise Castle Estate, Bristol, on 11.xi.1998, but the specimen was not determined to species. It is tempting to ponder that this was I. muscaeformis.
Conclusion
In Britain Issus muscaeformis is undoubtedly a climatically sensitive species, showing a clear western affinity, especially for areas of maritime influence. I muscaeformis may be more sensitive to winter weather than I. coleoptratus but it may live in greater exposure. The presently-known British distribution of I. muscaeformis correlates strongly with the mapped evidence of British mean annual temperature and climatic continentality (Elmes & Free, 1994). This shows that I. muscaeformis avoids areas of climatic continentality in Britain, occurring only in places where the difference between mean minimum January temperature and mean maximum July temperature is <15.5°C i.e. where the climate is equable. In Britain it occurs only in those places having more than nine hours of bright sunshine on >45 days of the year. This is a feature of all the more continental areas of the country where I. muscaeformis is absent and tangible proof that climatic equability is the key factor in its British distribution.
Any association with Yew would be significant in explaining the population focus in north-west England. Yew is known to have appeared in this area early on in the Holocene (Godwin, 1975) and this also is a species with a range determined exclusively by winter temperature. This focussed population of I. muscaeformis should be regarded as relict, and I. muscaeformis probably penetrated Britain relatively early on in the development of its post-glacial climax forests, indeed it is not an inhabitant of closed canopy woodland.
Although I. muscaeformis is usually cited as European in terms of its wider biogeography, this is an indication of range not of distribution. Hence, in Germany, as in Britain, it is highly restricted and is placed in Category 1 of the German 'Red List' (Nickel, Witsack & Remane, 1999). In Sweden it is also 'Red-listed' as vulnerable (Gärdenfors, 2000) but was not listed by Stoltze & Pihl (1998) for Denmark.
Acknowledgements
We thank Dr K.N.A. Alexander (Exeter); Mr J. Bowdrey (Colchester Museum); Sheila Brooke (Dunstable), for logistical assistance; Mr S. G. Dodd (Ash, Surrey), Mr W.R. Dolling (Hull); Mr A.P. Foster (National Trust, Swindon); Mr A.P. Fowles and the Countryside Council for Wales (Bangor); Dr E. Fründ (Scheeßel); Mr S. Hewitt (Tullie House Museum, Carlisle); Dr S. Judd (Liverpool World Museum); Dr P. Kirby (Peterborough); Mr J.W. Meiklejohn (Defford); Dr H. Nickel (Goettingen); Dr R. Niedringhaus (Oldenburg); the late Professor R. Remane (Marburg) and Dr M.R.Wilson (N.M.W., Cardiff). In particular we thank Mr M. Webb (B.M.N.H., London) and the Trustees of B.M.N.H. for making specimens available.
References
ALEXANDER, K.N.A., 1981. A second British record of Issus muscaeformis (Scrhk.) (Homoptera:Issidae) from North Lancashire. Entomologist's Monthly Magazine, 117:144.
BIEDERMANN R. & NEIDRINGHAUS, R., 2009. The plant- and leafhoppers of Germany. 409pp. ABV, Fründ.
DOLLING, W.R., 1982 (unpublished). Remarks on the British Issus (Hemiptera:Issidae). Unpublished draft.
DOLLING, W.R., 1991. The Hemiptera. Natural History Museum Publications, 274 pp. Oxford University Press.
ELMES, G.W. & FREE, A., 1994. Climate change and rare species in Britain. 28 pp. Natural Environment Research Council.
FERNÁNDEZ, Á., 2002. Visitor's guide to the Garajonay National Park, La Gomera. Ministerio de Medio Ambiente. 217pp.
FOWLES, A.P., 1994. The invertebrates of Wales: a review of important sites and species. 157 pp. J.N.C.C., Peterborough.
GÄRDENFORS, U., 2000 (ed.). Rödlistade arter I Sverige 2000. Artdatabanken, Uppsala.
GODWIN, H., 1975. The history of the British flora. pp. i-x,1-541.Cambridge University Press.
HOLZINGER, W.E., KAMMERLANDER, I. & NICKEL H., 2003. The Auchenorrhyncha of Central Europe 1. Brill.
KIRBY, P. 1992. A review of the scarce and threatened Hemiptera of Great Britain. UK Nature Conservation, 2. J.N.C.C., Peterborough.
LE QUESNE, W., 1960. Hemiptera: Fulgoromorpha. Handbooks for the Identification of British Insects, 2(3): 1-68. Royal Entomological Society of London.
NICKEL, H., 1997. Zur Verbreitung und Lebensweise einiger Zikadenarten in Niedersachsen und angrenzenden Gebieten (Homoptera, Auchenorrhyncha). Göttinger Naturkundliche Schriften, 4: 151-172.
NICKEL, H., WITSACK, W. & REMANE, R., 1999. Rote Liste der Zikaden Deustchlands (Hemiptera, Auchenorrhyncha), Habitate, Gefahrdungsfaktoren und Anmerkungen zum Areal. Beiträge zur Zikadenkunde, 3: 59-78.
OSSIANNILSSON, F., 1978. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark Part 1. Introduction, infraorder Fulgoromorpha. Fauna Entomologica Scandinavica, 7(1): 1-222.
PAYNE, K., 1979. Auchenorrhyncha of Gait Barrows National Nature Reserve near Arnside, Lancs. (SD/47). Entomologist's Monthly Magazine, 114: 210.
QUARTAU, J.A., In: Borges, P.A.V., Abreu, C., Aguiar, A.M.F., Carvalho, P., Jardim, R., Melo, I., Oliveira, P., Sérgio, C., Serrano, A.R.M. & Vieira, P. (eds), 2008. A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. 438 pp. Direcção Regional do Ambiente da Madeira and Universidade dos Açores, Funchal and Angra do Heroismo.
REMANE, R., 1985. Vorläufige Anmerkungen zur Evolution und Speziation der Gattung Issus F. auf den Mittelatlantischen Inseln (Kanaren, Madeira) (Homoptera, Auchenorrhyncha, Fulgoromorpha, Issidae). Marburger Entomologische Publikationen, 1(10): 1-168.
STOLTZE, M. & PIHL, S. (eds), 1998. Rødliste 1997 over planter og dyr i Danmark. Miljø og Energiministeriet og Danmarks Miljøundersøgelser og Skov og Naturstyrelsen.
THOMAS, J., 2006. Issus muscaeformis (Schrank) (Hemiptera: Issidae) a speciality of North West England. Journal of the Lancashire & Cheshire Entomological Society, 130:51-52.
WHITEHEAD, P.F., 2010. Dryinus collaris (L., 1767) (Hym., Dryinidae) new to Worcestershire. Entomologist's Monthly Magazine, 146:26.
ZENNER, G., STÖCKMANN, M., NIEDRINGHAUS, R., 2005. Preliminary key to the nymphs of the families and subfamilies of the German Auchenorrhyncha fauna (Hemiptera, Fulgoromorpha and Cicadomorpha). Beiträge zur Zikadenkunde, 8: 59-78.
P.F.W., Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH, England, U.K. e-mail:paul@thewhiteheads.eu
R.S.K., The Old Black Bull, Carthorpe, Bedale, North Yorkshire DL8 2LD, England, U.K.
Footnote.
For the first time in this study, L2 Issus coleoptratus nymphs were observed on ivy at Malvern, Worcestershire, during October 2010, bearing markedly elongate linear grey waxy caudal appendages almost the length of the abdomen. It is presumed that these rarely observed and seldom-cited structures, reminiscent of those of cixiid nymphs, are readily shedlost early in life and seldom observed.