A multi-scalar analysis of habitat characteristics associated with the Noble Chafer beetle Gnorimus nobilis in south Worcestershire
Fred Windsor
Introduction
Anthropogenic activity and intensification of agricultural production have facilitated a decline in species diversity across Europe throughout the 21st century (Pimm et al., 1995). A key driver of this change was the post-WWII transition from agricultural subsistence to intensive mass production instigating dramatic shifts in the character of agricultural landscapes (Bignal and McCracken, 1996; Robinson and Sutherland, 2002; Stoate et al., 2009). Contemporary management focuses on achieving the maximum profit per hectare (Rounsevell et al., 2003), which in many cases promoted the adoption of alternative forms of more profitable agriculture (Paracchini et al., 2007; Sharples, 2007; Barker et al., 2011). Orchard habitats within the UK saw a paradigm shift away from traditional management (Koziell, 2000; Robertson and Wedge, 2008), concentrating activity in relatively small pockets of land, thousands of hectares smaller than previously (Barker et al., 2011). Furthermore, altered management facilitated an increased utilisation of pesticides, herbicides and fungicides which are directly deleterious to wildlife (Epstein et al., 2001; Barker et al., 2011). Although traditional management still persists within orchard agriculture (Cloke and Jones, 2001; Duarte et al., 2008), a loss of favourability continues to reduce its utilisation (Tscharntke et al., 2005). This is detrimental for biological diversity as traditionally managed agricultural and arboreal habitats are extremely species rich (Berg et al., 1994; Bignal and McCracken, 1996; Alexander, 1999; Ranius, 2001; Plieninger et al., 2006; Orłowski and Nowak, 2007; Müller et al., 2008; Bailey et al., 2010).
The transition away from traditional, low intensity management has critically affected the volume of decay habitats, which are declining throughout Europe (Grove, 2002a; Ranius and Fahrig, 2006; Ranius, 2007). Decreasing decay has negatively impacted many organisms, especially those within the saproxylic habit (Nilsson, 1997; Ranius and Wilander, 2000; Brown and Schmitt, 2001; Löfman and Kouki, 2001; Benton et al., 2002; Jonsell and Nordlander, 2002; Similä et al., 2003; Philpott et al., 2008; Ranius and Roberge, 2011). Saproxylic species are defined as organisms which possess a distinct association with either standing or moribund decaying wood and/or the products of such decay (Speight, 1989; Alexander, 2008a). The saproxylic habit encompasses entire families (Webb et al., 2008) and occupies a distinct range of habitats (Grove et al., 2002a). Thus it appears imperative that undisturbed sites of old-growth orchards are conserved in order to maintain saproxylic community integrity (Niemelä, 1997).
Beetles (Coleoptera) are an especially abundant and diverse order, which often significantly contribute to the aforementioned diversity of agricultural habitats (Bouchard et al., 2009; Groombridge, 1992). The Noble Chafer beetle Gnorimus nobilis is a visually conspicuous saproxylic species (Krikken, 1984: Whitehead, 2003; PTES, 2008a), endemic to large areas of Europe. Its dominant range spans the north-west of the continent with less frequent distributions extending southwards (Osborne, 1974; Tauzin, 2000). Within the UK the anthropogenic influences have altered the structure and composition of arboreal habitats (Wilson, 2002; Hopkins and Kirby, 2007), in turn fragmenting decay habitat (Hannah et al., 1995). This has significantly altered the distribution of G. nobilis (Alexander, 2008b). Contemporary populations exist within central to southern England and although covering a relatively broad geographical area, limited dispersal restricts G. nobilis to a finite number of waning habitats (Cooter et al., 1991; Alexander, 2008b). Subsequently G. nobilis, as well as other saproxylic species, is vulnerable and at risk of extinction (Smith, 2003; Evans and Whitehead, 2005; JNCC, 2007; Macdonald et al., 2007; Alexander, 2008b; Nieto and Alexander, 2010; JNCC, 2010; Sebek et al., 2013).
Regions associated with G. nobilis are concomitant with traditionally managed orchard horticulture (Robertson and Wedge, 2008), which appears to be the dominant habitat of G. nobilis within the UK (Alexander, 2002; Whitehead, 2003; Alexander, 2008b; PTES, 2008a; Lush et al., 2009; Alexander and Bower, 2011). Nevertheless, alternate, non-horticultural populations have been recorded (Alexander, 2010). Traditional orchard habitat is defined as: “… [sites] where at least 5 fruit trees must be present with no more than 20m between their crown edges.” (Burrough and Robertson, 2008, pp. 27). Management is vital (Kirby et al., 1995). This encompasses three strategies: (1) The generation of low canopy densities (Robertson and Wedge, 2008); (2) the complete absence of artificial pesticides and fertilisers (Herzog, 1998; Cordrey et al., 2008); and (3) periodic mowing or grazing (Burrough and Robertson, 2008). This in turn allows for a large quantity of decay habitat (Whitehead, 2003; Schroeder et al., 2007), suitable for saproxylic species.
The association with orchard agriculture, however, only occurred contemporaneously. Osborne (1974) showed that during the early Flandrian period G. nobilis populations were associated with pioneer deciduous woodland. Therefore a transition from deciduous woodland to orchard habitat has transpired. This is most likely due to anthropogenic disturbance within deciduous woodland reducing decay quantity, and therefore suitability.
In spite of globally diminishing decay habitats, the UK maintains the largest reserves of old-growth habitat in northwest Europe (Alexander, 1998). Nevertheless, the status of such habitat remains in the balance, with increasing orchard vulnerability (Marshall, 2008; Widdicome et al., 2008) coinciding with losses of old-growth habitat diversity (Sippola et al., 2002; Grove, 2002d). Therefore further research is vital for supporting existing conservation whilst further increasing the preservation of old-growth habitat (Whitehead, 1992; 1997).
This exploratory study is concerned with the unique habitat characteristics produced by traditional orchards with regards to G. nobilis presence throughout the landscape of south Worcestershire. Within this assessment a previously unexplored scale of inhabitance is examined, allowing for further ecological knowledge regarding G. nobilis to be acquired. This provides a novel contribution to a still relatively under-researched field of British entomology. The implications of this research are particularly important for the continuation of traditional management within the landscape, which in turn appears to facilitate the persistence of this species within the UK.
Scientific Background
Literature regarding G. nobilis is sparse, with relatively few studies explicitly analysing the characteristics of occupied orchards. Therefore, the analysis of research regarding similar saproxylic beetle species provides direction within this study. Habitat characteristics of inhabited sites are of particular interest due to the unexplained transition from deciduous woodland and the explicit absence of G. nobilis from intensively managed sites (Alexander, 2008b).
Scale is important to consider when assessing habitat biodiversity (Nilsson et al., 2001; Murphy and Lovett-Doust, 2004; Bergman et al., 2012). Environmental characteristics transcending multiple spatial scales have been observed affecting the distribution of organisms (Elith and Leathwick, 2009) and specifically arthropods (Jonsell et al., 1998; Meggs et al., 2003; Holland et al., 2004; Cardoso et al., 2009). These characteristics can be segregated based on the spatial scale over which they operate (Holland et al., 2005) as well their influence on species distribution (Mackey and Lindenmayer, 2001; Chase, 2007; Lindo and Winchester, 2009). Within the literature two distinct categories are observed: Habitat/site and landscape. Within these broad categories several discrete sub-classifications are observed.
Therefore it can be seen that a variety of spatial scales need to be analysed so as to comprehensively detail the population distribution of saproxylic beetles (Bergman et al., 2012). Complexity is provided by the holometabolous nature of G. nobilis, which exhibits several instars throughout development (Whiting, 2002; Whitehead, 2003). The developmental stage of an organism alters how environments and resources are perceived (Murphy and Lovett-Doust, 2004). Due to this an appreciation of the requirements of G. nobilis throughout its multiple developmental stages will be presented throughout this dissertation.
Landscape Scale
Landscape characteristics
The landscape surrounding traditional orchards has received little attention, with regards to land-use. However, land-use may be significant as G. nobilis adults have been observed nectaring on Apiaceae (Whitehead, 2003), and may rely on open landscapes, devoid of obstacles in order for successful dispersal. Not only is the contemporary land-use important, the longevity of landscape features appears significant for G. nobilis (Alexander, 2008b), with the temporal prerequisites of decay production limiting saproxylic species to well-developed habitats (Cardosa et al., 2009).
Landscape characteristics create unique interactions, transcending multiple scales (Stoner and Joern, 2004) which may introduce complexities, some of which are not yet fully understood. Thus through an analysis of multiple scales, an in depth picture of interactions can be constructed. An assessment of structural and functional connectivity (Collinge, 2000; With et al., 1999) will elucidate the relative importance of the landscape; potentially highlighting the downward influences impacting the suitability of local habitat within the wider landscape matrix (Sirami et al., 2008). Such multi-scalar interactions have not been assessed for G. nobilis but have significantly influenced other arthropod species (Fetridge et al., 2008).
Habitat/Site scale
Orchard characteristics
G. nobilis is explicitly absent from intensively managed orchard stands (Whitehead, 2003; Alexander, 2008b), thus specific influences at this scale may provide an insight into significant habitat characteristics. The nature of orchard sites modifies local habitat climate, e.g. temperature and wind speed; thereby influencing species presence (Jeanneret et al., 2003; Frank et al., 2009; Chiari et al., 2012; Lachat et al., 2012). It appears high density cultivation within intensive orchards makes them unsuitable for G. nobilis through these mechanisms (Atkinson and Winnall, 2008). High density canopies create shade and prevent adequate sunlight penetration (Franklin et al., 2002). Thus, cool, damp microclimates are produced, providing conditions that are highly unfavourable for saproxylic species dependent on radiative heating for incubation (Alexander, 2008b) and the production of decay products, on which larvae feed (Zdenĕk et al., 2012). These effects are exacerbated for G. nobilis as it is a particularly temperature sensitive species (Renault et al., 2005). However, as of yet an examination of relative density within traditional orchards is absent. Previous research regarding G. nobilis has be predominantly qualitative and observational, thus quantification of relationships may provide more substantial evidence. Habitat volume, density and heterogeneity significantly influence the colonisation of habitat (Murcia, 1995; Similä et al., 2006), with important effects including edge-area ratios (Ewers et al., 2007) and inter-habitat connectivity (With and Crist, 1995). The assessment of multiple orchard factors within this study looks to encapsulate some of these effects in a quantifiable manner.
Tree characteristics
Progressing vertically down through the spatial hierarchy, we reach individual tree characteristics. The production and availability of local habitat, within the wider context is vital for determining the distribution of species (Martikainen, 2001). This is especially significant as the high diversity associated with arboreal habitats is generally created by site specific conditions (Ranius, 2006). These characteristics, however, are not always observed providing a significant influence for saproxylic species (Similä et al., 2002). Although, in the case of G. nobilis local habitat influences appear dominant (Lush et al., 2011; Alexander 2008b; Hurt and Burrough, 2009). Many characteristics at this scale have been assessed, although conclusions are generally inconsistent; with studies assessing both tree species and girth tending to contradict one another, with few regular trends emerging (e.g. Alexander, 2008b; Lush et al., 2009; Alexander and Bower, 2011).
G. nobilis and other saproxylic species are particularly reliant on the suitability of decay habitat (Grove, 2002c; Alexander, 2003; Jacobs et al., 2007; Lush et al., 2009; Ranius et al., 2009a; Alexander and Bower, 2011). Yet previous research has only focused on G. nobilis populations with regards to the presence or absence of this resource. Results, however, indicated that the absence of G. nobilis from intensively managed orchards may be a product of low heartwood and decay accumulation (Atkinson and Winnall, 2008; Lang, 2001; Robertson and Wedge, 2008).
A further defining feature of previous research is that only inhabited trees have been assessed with little investigation of the differences between colonised and un-colonised habitat. Comparisons between these habitats may be particularly successful in the case of G. nobilis as highly specialised saproxylic species may be distributed across relatively few suitable host trees (Davies et al., 2008). Therefore characteristics of inhabited sites may be concise in comparison to unsuitable features within uninhabited sites.
Microhabitat characteristics
Microhabitat characteristics refer to the conditions found within individual bole features as defined by Michel and Winter (2009) and Read (2000). They are vital in maintaining the diversity and distribution of species within arboreal ecosystems (Rotheray, 2013; Larrieu et al., 2012) and have been shown to influence the distribution and abundance of saproxylic species across habitats (Tahvanainen, 1972; Jonsson et al., 2005; Wermelinger et al., 2007). Such variations enable greater local variation than larger scales (Jonsson et al., 2005). Although, some characteristics provided by vegetative micro-features are less dynamic and can stabilise microclimatic variability (Rukke and Midtgaard, 1998; Molina-Montenegro et al., 2009). No work has been completed on microhabitat characteristics with regards to G. nobilis, yet as seen, similar species are influenced at this scale. Research suggesting weak or redundant relationships between micro-scale characteristics, diversity and abundance of saproxylic species (e.g. Økland et al., 1996; Siitonen and Saaristo, 2000), are generally derived from high dispersal strength of the species (Økland et al., 1996). In the case of G. nobilis observational research suggests that it may be a poor disperser (Whitehead, 2003), and thus local features may dominate.
Research questions
Considering previous research on G. nobilis and comparisons with other similar species several themes are highlighted. The overarching question considered by this dissertation is whether G. nobilis displays associations with specific habitat characteristics throughout the south of Worcestershire. This encompasses analysis of four distinct scales: 1) Landscape; 2) Orchard; 3) Tree; and 4) Microhabitat.
Across these four scales there are several research objectives:
Assess the differences between inhabited and uninhabited orchard characteristics within south Worcestershire.
Assess the relative influence of each distinct spatial scale on the distribution of G. nobilis within south Worcestershire.
Assess the relative influence of individual habitat characteristics on the distribution of G. nobilis within south Worcestershire.
Methodology
Study area
South Worcestershire has long been recognised as a stronghold for horticulture (Robinson, 1983), providing suitable habitat for G. nobilis populations; confirmed by records of G. nobilis dating back to 1945 (Evans and Whitehead, 2005). Historical records of G. nobilis indicators and sightings within Worcestershire are prevalent throughout south Worcestershire, maintaining a particularly large density of contemporary records.
The sites analysed within this dissertation are dispersed across central-south Worcestershire, encompassing approximately 25km2. Sites were provisionally selected using the Natural England traditional orchard habitat survey, which was part of a wider habitat cataloguing scheme instigated by Natural England in 2011 (Natural England, 2012). Remotely sensed data from historical maps and high resolution photogrammetry was collected utilising a methodology similar to that of Warner and Steinmaus (2005) and García Torres et al. (2008). This facilitated the production of a traditional orchard habitat map (Natural England, 2012). Remotely sensing land-use characteristics is a technique becoming increasingly feasible and widely applicable due to its high accuracy and wide spatial footprint (Turner et al., 2003). However, errors are associated with this method (Nagendra et al., 2013); therefore ground-truthing of selected sites was also completed. During this truthing some original sites proved to be incorrectly identified; in addition sites which were also considered to be traditionally managed, but not identified by the survey were encompassed.
Data collection
Sampling Framework
Data collection occurred from late June to mid-July. This time period allowed for a sample encompassing the time of highest activity for G. nobilis adults (Whitehead, 2003). The time of sampling, however, is inconsequential for larval identification as frass produced by G. nobilis remains in situ for extended periods of time, allowing for the year round identification of G. nobilis populations (Hurt and Burrough, 2009). Although not directly measured, observations of adults in orchards aided the location of potential populations.
The number of trees sampled in each orchard was not uniform. The minimum number of trees suitable was derived from Burrough and Robertson (2008); providing a lower boundary of five. An initial habitat survey was completed for all orchards; within this, the basic orchard condition was assessed alongside a calculation of tree numbers. Due to the quantity of trees in some orchards a sub-sample was completed. In smaller orchards however all trees were assessed.
Sub-sampling consisted of a systematic spatial framework, which provides comprehensive and unbiased samples of red-listed populations (Hedgren and Weslien, 2008). The framework was derived from the quantity of trees within each site; produced from the initial habitat survey. This allowed for the calculation of a multiplicative factor which enabled a method to sample 40 trees. This schedule spatially segregated each orchard into quarters (e.g. Timmer et al., 1988), within which ten trees were assessed. Due to the high heterogeneity and variability of decay habitat (Pyle and Brown, 1999) a representative sample of microhabitats was also completed. The sample size was dependent on relative volume of decay habitat within each orchard. Therefore in well-developed orchards, with large volumes of microhabitats, sample sizes were larger. Overall, 20 orchards were sampled with a total of 478 trees and 122 microhabitats.
G. nobilis identification
Contemporarily, there is no completely unbiased sampling technique which accurately encapsulates distribution, abundance and richness of saproxylic communities (Ranius and Jansson, 2002). In order to accurately detail the population distribution of near-threatened and endangered species large sample sizes of more than 200 individuals (adult lifestages) are generally required (Martikainen and Kouki, 2002). Samples of this magnitude are potentially deleterious for often vulnerable G. nobilis populations. Furthermore, trapping methods do not always provide an accurate spatial sample of saproxylic populations (Ranius and Jansson, 2002; Alinvi et al., 2007; Jonsell and Weslien, 2003; Siitonen, 1994; Økland et al., 1996). Lastly, G. nobilis adults are notoriously elusive (PTES, 2008a). Therefore it was unfeasible to sample adult populations within this study.
Nonetheless, comprehensive methodological frameworks exist for sampling Coleopteran larvae. Larval instars of saproxylic species are obligate to decaying wood (Webb et al., 2008); providing a less dynamic and more reliable indicator of saproxylic beetle populations (Ranius and Nilsson, 1997). Furthermore, larvae sampling can too provide accurate samples of population structure and dynamics (Siitonen, 1994). Therefore within this dissertation the identification of larval populations took place. The dominant method of identifying G. nobilis larvae is microhabitat sampling. This method relies upon the identification of frass; a characteristic excrement deposited in the wake of feeding larvae (Hurt and Burrough, 2009). This method is based on the fact that G. nobilis is the only species to produce this distinctive frass within the traditional orchard habitat (Whitehead, 2003; Hopkins and Kirby, 2007; Macdonald et al., 2007). Beetle fragments were also used alongside frass as indicators of inhabitance (Ranius, 2000).
Multiscalar characteristics
Multiple techniques were used in order to collect characteristic data. The primary method of landscape analysis utilised a GIS. Remote sensing of landscape characteristics within 1km of sites was completed, with the manual digitisation providing the basis for land-use classification. Altitude and aspect were calculated for each site using a DEM derived from satellite altimetry data (Sharma and Panigrahy, 2007). The majority of qualitative data was compiled using likert scales with descriptive characteristics categorising variables.
The assessment of orchard characteristics took place predominantly through a semi-quantitative habitat evaluation (e.g. Tikkanen et al., 2007). Tree density was calculated using the orchard habitat perimeters provided by Natural England (2012) and/or GPS data collected during sampling.
Tree characteristics were composed of a series of quantitative variables. Recording the location of inhabited trees/orchards is a necessary procedure for G. nobilis surveying, allowing for representations of distribution within habitats (Dominy and Duncan, 2001). Tree species identification through phenotypic observation is a long and often difficult process (Wünsch and Hormaza, 2002); accordingly, sites with little information received taxonomic identification to genus. Inhabited trees were classed as those which provided accessible sample features, with the presence of G. nobilis indicators.
G. nobilis larvae reside deep within heartwood, therefore the direct sampling of microhabitat characteristics could be extremely damaging. Thus easy to measure, external features reflecting the conditions within microhabitats were utilised. Primary measurements comprised two major microclimatic variables identified by Bässler et al. (2010); temperature and humidity. External and internal paired readings were recorded; providing a comparison, and accounting for macro-climatic fluctuations. A further temporal study of temperature utilising a Tinytag™ monitor at a specific inhabited microhabitat within Tiddesley orchard. Qualitative measurements focused on the composition of wood mould, again utilising likert scales.
Results
Data collected from the field was first explored to allow for the avoidance of statistical errors related to the utilisation of incorrect statistical analyses and other common problems. A framework similar to that of Zuur et al. (2010) was used in order to assess data preceding statistical analysis. Normality and collinearity were of particular concern for parametric tests, in particular logistic regressions completed on each scale of habitat required independent variables. Correlation matrices were produced in each instance; highlighting interconnections between variables which may have been assumed as discrete. The removal of variables significantly related to one or more other variables excluded multicollinearity from the model whilst preserving an explanation of inhabitance.
Ten out of the twenty sampled sites displayed indicators of G. nobilis presence. Sites were significantly dispersed across the landscape, however, a number of inhabited and uninhabited sites are located within close proximity to one another.
Landscape characteristics
Ambiguity was found amongst some quantitative measures of landscape characteristics. Orchard aspect and altitude were not significantly different between inhabited and uninhabited sites (t=-1.389, df=9, p=0.198 and t=-0.183, df=9 p=0.859 respectively). Further to this, there was no significant correlation between said factors and the abundance of G. nobilis indicators within inhabited sites (rs=0.286, p=0.221 and rs=-0.14, p=0.954 respectively). Therefore it can be seen that topographical characteristics maintain little importance in G. nobilis distribution.
An analysis of land-use surrounding sites provided significant relationships with the presence of G. nobilis. The dominant factor was the volume of orchard habitat surrounding sites; occupied and unoccupied sites differed significantly (t=-2.985, df=9, p=0.15). Not only was the percentage of surrounding orchard land-use important for distribution of G. nobilis over sites, it also appeared to affect the abundance of inhabited trees, with a positive relationship, however this was discovered to be statistically insignificant (rs=4.16, p=0.068). Both urban and open ground volume displayed a negative (although insignificant) influences on abundance (rs=-0.340, p=0.198 and rs=-0.345, p = 0.137 respectively). Remotely sensed data although subjective, exhibited interesting relationships between landscape characteristics and G. nobilis presence, which supported statistical data.
The landscape surrounding a particularly suitable orchard (site 14) has high densities of both mixed woodland and orchard habitats (16.14 and 27.18 ha respectively), confirmed by a previous statistical correlation derived from these digitised land-use characteristics. Representations of openness allowed for a combined analysis with a finer scale characteristic, orchard size. With larger sites it appears there is a observable positive relationship with openness, however smaller sites appear to display a negative relationship with open ground.
The orchard sites sampled were generally fragmented and isolated; not linked with suitable habitat across the Worcestershire landscape. Although, some inhabited sites are well connected with orchard land-use and inhabited orchards, albeit over a relatively small scale. Distinct barriers inducing fragmentation were apparent across the landscape.
Conurbations were observed in close proximity to the majority negative sites. Historical data, helped to display the increasing urban sprawl derived from the increase in industry and agricultural within the Midlands (Wrigley, 1985); which appears to negatively impact the distribution of G. nobilis with sites located in close proximity generally remaining uninhabited, with a strong positive correlation between distance from urban areas and G. nobilis populations (rs=0.551, p=0.012). Orchard age was also inferred from historical maps. Only inferences can be made from such data, however, it appears that sites with historically persistence orchard land-use (e.g. where orchards have been present since the 1885) maintain contemporary populations of G. nobilis. Roads were too a dominant feature surrounding uninhabited sites. Three inhabited sites were discovered to the north of the A44, yet sites in close proximity, but to the south of the road were uninhabited.
Due to several significant relationships found between landscape characteristics and G. nobilis distribution a binary logistical regression was completed to assess which characteristics most likely predicted occupancy of sites. The regression displayed one significant predictor; the percentage of orchard land-use surrounding sites. This increased the predictive power of the null model (Nagelkerke r2=0.421); presenting a greater likelihood of colonisation with increasing volumes of orchard in the surrounding landscape.
Orchard characteristics
In general, orchard characteristics did not significantly influence G. nobilis distribution. The diversity of orchard tree species was not significantly different between inhabited or uninhabited sites (t=-0.889, df=9, p=0.397) and only a weak, insignificant correlation existed with the presence of G. nobilis (rs=-0.059, p=0.806). Tree density, quantity and habitat size displayed relatively weak and insignificant relationships with the presence of G. nobilis (rs=0.047, p=0.845 and rs=0.388, p=0.091, rs=0.373, p=0.105 respectively). Having completed Q-Q plots environmental variables, it was found that the quantity of trees was abnormally distributed and thus a logarithm was utilised to induce normality.
The form of management found within the orchard, though not displaying any statistical significance, appeared to play a vital role in extremes situations. Sites which were overgrown, with high abundances of species such as Ivies hedera were completely uncolonised by G. nobilis (Site 3 and 4), whereas sites with traditional management, either through periodic low intensity mowing or grazing appeared more suitable, with 60% of traditionally mown or grazed sites being occupied.
Tree characteristics
The characteristics found within tree habitat appear to play a more significant role in G. nobilis colonisation. Several relationships were found between tree characteristics and G. nobilis distribution. Prunus domestica facilitated the greatest G. nobilis populations (29.98% of records), relatively high compared to that of Prunus insititia (8.69%), Pyrus domestica (7.21%), and Malus domestica (1.51%). However a large proportion of inhabited data were collected from Site 11, composed of solely P. domestica. Therefore other factors influencing distribution may be obscured by this observation.
Both tree height and girth were found to be insignificantly different between inhabited and uninhabited sites (t=0.046, df=55, p=0.963; and t=0.776, df=55, p=0.447 respectively). No indicators of G. nobilis were discovered in tree girths less than 0.45m; with the mean tree girth per orchard showing that occupancy occurred in sites averaging between 0.5m and 1.5m. The majority of uninhabited records were provided by living trees early in their decomposition, whereas inhabited trees typically displayed heavily decaying boughs and trunks (75% of records), with 36.6% of moribund trees inhabited. Therefore there appears a greater likelihood of inhabitance in latter, mature stages of decomposition. Furthermore, orchards with greater densities of these late successional trees exhibited higher likelihood of colonisation (73.29% late successional trees in occupied sites). Not only was the stage of decay important so too was the quantity, with the density of decay microhabitats displaying a significant difference between inhabited and uninhabited trees (t=-13.490, df=81.944, p=<0.001).
A binary logistical regression was performed, utilising particularly significant habitat characteristics. The model showed that the density of microhabitats and tree species were significant factors influencing colonisation of tree habitats. The density of microhabitat increased the probability of G. nobilis presence by over three times in trees with fine substrate (exp b= 3.707). Within the category of tree genera Malus displayed the greatest significance, yet did not drastically alter the probability of presence. The model provided a reasonable fit overall, and allows for adequate prediction of inhabitance (Nagelkerke r2=0.417).
Microhabitat characteristics
Monitoring temperature provided an insight into the microclimatic stability produced within microhabitats and orchards to a greater extent. The range of measurements found in internal temperatures was significantly less than that of external temperature (Internal=14.80, External=15.70). This is supported further by measurements recorded from an individual tree feature within Site 11. However, a distinct diurnal relationship between internal and external temperatures persists. Within the morning hours (6:00-12:00) internal temperature falls below that of external temperature.
The range of humidity recorded was far more variable between individual features (Internal=47.80 and External=32.30). Nevertheless, higher mean moisture levels were recorded internally (mean=61.06%) compared to the externally (mean=55.48%). Therefore it can be seen that distinct microclimates are created internally within trees. An independent Mann-Whitney U Test was completed for both temperature and humidity within inhabited and uninhabited features. This showed significant difference between the two data sets, thus rejecting the null hypothesis (Temperature: p=<0.001; Humidity: p=0.044). This highlights the difference between microhabitats found within inhabited and uninhabited orchards.
Feature orientation and depth of rot helped to display potential microclimatic conditions. However, the orientation of microhabitat entrances was not significantly different between inhabited and uninhabited sites (t=-1.458, df=120, p=0.147). Therefore the orientation of features appears insignificant for G. nobilis distribution. A subjective analysis does appear to show that easterly facing features are less well inhabited; nevertheless the variability of results limits the significance of such interpretations. On the other hand the depth of the feature does appear to play an important role in determining distribution. Inhabited trees has significantly deeper features than those which were uninhabited (t=-8.728, df=120, p=<0.001).
Internal temperature and depth of rot were collinear with several other variables and were thus removed from the logistic regression model. Wood mould density was the dominant factor influencing the occurrence of G. nobilis. However, temperature also appears to influence distribution, with higher temperatures providing a greater likelihood of G. nobilis presence. The Nagelkerke r2 value indicates that the model accurately predicts 70.7% of variation, thus strongly predicting presence. Humidity was also implicated within the model, however, results were unaccountably variable throughout the sampling period, and thus were discounted as a significant variable influencing G. nobilis presence.
Discussion
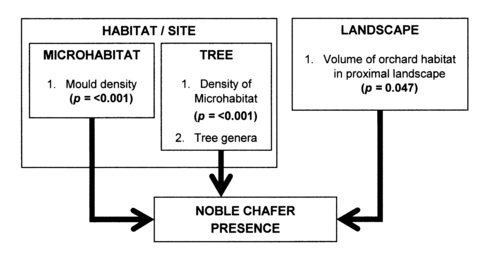
The principal objective of this dissertation was to elucidate differences in multi-scalar habitat characteristics between orchards both inhabited and uninhabited by G. nobilis in order to provide evidence concerning variables determining presence. These variables are essentially located at either landscape or habitat scales (Mazerolle and Villard, 1999; Stoner and Joern, 2004; Tikkanen et al., 2006). Within this study three separate spatial scales were classified within habitat: 1) microhabitat, 2) tree, 3) orchard; with a single scale representing the landscape (01).
Several significant characteristics were displayed at both habitat and landscape scales (Figure 5.1). The fact that specific characteristics are found within inhabited sites suggests that there is distinct difference between the characteristics of orchards exhibiting G. nobilis presence and absence. When compared, habitat characteristics explained a greater amount of probability associated with the presence of G. nobilis this helps to answer the second research question proposed within this study. Relationships at habitat level were strongest at microhabitat scale, shown by the high prediction success of the microhabitat characteristics (70.7%). Nevertheless landscape influences were observed influencing the presence of G. nobilis; with the volume of orchard habitat in the landscape provided relatively accurate predictions of presence. The results displayed within this study highlight several key characteristics located at both scales suggesting a balance between these scales determines the presence of G. nobilis. Each scale is discussed in turn, proceeding with the most influential.
Habitat / Site scale
Microhabitat characteristics
Binary logistic regression displayed a particularly accurate model for the prediction of G. nobilis presence, derived predominantly from microhabitat characteristics. Within this model the density of the wood mould was identified as being a strong predictor. Output showed that G. nobilis was present in trees which displayed lower densities of wood mould. A reduction in substrate size and density over time indicates the presence of significant processing and decomposition within habitats (Mattson et al., 1987); thereby inferring that a prerequisite of G. nobilis presence is well processed substrate within microhabitats. As of yet little work has been completed for G. nobilis at a microhabitat scale, yet similar relationships with fine substrate have been detailed by several studies of saproxylic species (Nilsson and Baranowski, 1997; Abrahamsson and Lindbladh, 2006). From such studies it is apparent that certain species, and potentially G. nobilis, require pre-processing in order for assimilation of nutrients (Kaila et al., 1994; Butler et al., 2002; Jansson et al., 2009).
Wood mould density and processing is influenced by several factors. The wood mould density within this study was significantly correlated to the successional stage of the tree habitat (r²=0.539, p=<0.001). This relationship corroborates findings from Nilsson et al. (2002) and Ranius (2003), who stated that dead wood is a product of stand age, low management and low disturbance. Colonisation of fungi was not analysed within this study, yet it may be important, with observations suggesting microorganisms assist processing of decomposing wood material, thereby reducing wood mould density (Jonsell et al., 2005; Djupström et al., 2010). In some cases fungal processing is so significant co-variation has been suggested (Similä et al., 2006). Therefore further analysis of fungal composition may provide relationships with G. nobilis.
Enhanced fining of decomposition was also related to rot depth within the orchard microhabitat (rs=0.42, p=<0.001) and although not included as a variable within logistic regression, rot depth displayed a strong positive relationship with G. nobilis presence. G. nobilis larvae specifically are observed habituating rot deep within the heartwood of the tree (Alexander, 2008b). Substrate characteristics have yet been quantified for G. nobilis, however, they are important for several saproxylic species (Jacobs et al., 2007). The combinations of density and depth observed within this study have been identified previously for similar species (Nilsson and Baranowski, 1999). It is therefore likely that multiple associated substrate characteristics influence the presence of G. nobilis.
Microclimatic characteristics established within microhabitats also influenced presence of G. nobilis within this study. Likelihood of presence coincidentally increased with temperature; furthermore, there was significant difference between temperatures in inhabited and uninhabited microhabitats. Research completed by Renault et al. (2005) indicates that G. nobilis exhibits a low cold tolerance, therefore supporting this finding. Moreover, temperature regulation is important within this study; indicating the low variability of microhabitat temperatures compared to external, macroscale fluctuations. The regulation of temperatures at this scale is seen to increase species abundance in saproxylic assemblages (Wright, 1983; Lindo et al., 2008; Lachat et al., 2012) as well as influencing the presence of G. nobilis.
The regulation of moisture is also essential for some species (Jonsell et al., 2001; Chiari et al., 2012). However, a complicated relationship between microhabitat moisture and G. nobilis was present. Repeat measurements display greater internal variation compared to external conditions; although data from an extended period displayed a high mean with relatively low deviation (68.22 ± 13.43%). Furthermore, moisture was utilised in a model predicting G. nobilis presence. As shown by Hjätlén et al. (2007) moisture content of wood significantly affects the rate of decomposition as well as the composition of substrate; both of which have been previously shown to influence G. nobilis presence within this study. The regulation provided by microhabitats may have indirectly affected G. nobilis facilitating optimum substrate conditions, whilst reducing mortality from extreme climatic variation.
Orientation of microhabitat features often influences microclimate, and therefore has been observed displaying a relationship with the presence of saproxylic species (Ranius and Nilsson, 1997; Ranius and Wilander, 2000; Ranius, 2002a). Nevertheless there was no association displayed between G. nobilis and the orientation of features in this study. This may represent the depth at which G. nobilis larvae are found within heartwood decay (Whitehead, 2003), allowing for reduced external influences. This is supported by the absence of relationships between G. nobilis and other external influences such as macroscale temperature and humidity. A further possible explanation lies at a broader spatial scale; the tree density of traditional orchard cultivation. Studies completed previously analyse the presence of saproxylic species in closed canopy, dense woodland, which generally exhibited low light penetration; creating significant implications for orientation on radiative heating. The setting of this study allows for greater light penetration due to regulated planting densities (Alexander, 2008b), therefore potentially reducing the influence of orientation.
Previous studies have generally neglected characteristics at microhabitat scales. However within this study microhabitat characteristics significantly influenced G. nobilis presence. Therefore, further study of is essential, with strong preliminary evidence suggesting relationships at this scale.
Tree characteristics
A key result of this study was the absence of significant relationships between tree height, girth and G. nobilis presence. The same result was displayed by Alexander and Bower (2011, Table 1) with a similar range of girths inhabited. Nevertheless, due to previously inconsistent findings and the significant obligation to specific tree girths displayed by many European saproxylic species (Økland et al., 1996; Siitonen, 2001; Lindhe et al., 2005; Gibb et al., 2006; McGeoch et al., 2007; Buse et al., 2008; Müller and Bütler, 2010; Horák et al., 2010), it was expected that a relationship might exist. A range of potential relationships exist from studies of similar species (Martikainen et al., 2000; Ranius and Jansson, 2000; Schiegg, 2001; Ranius, 2002a; Hammond et al., 2004; Penttilä et al., 2004; Yee, 2005; Buse et al., 2007; Oleska et al., 2007; Wu et al., 2008). However, no clear relationship with girth was identified for G. nobilis (cf. Alexander and Bower, 2011). Conversely, no distinct lower boundary was observed as in their study, yet they did recognise complications preventing the identification of consistent relationships, e.g. the influence of tree species (Weedon et al., 2009).
The density of microhabitat and thus availability of decay substrate significantly affected the presence of G. nobilis. The significance of this relationship has been previously highlighted (Whitehead, 2003; Alexander. 2008b; Lush et al., 2009). Grove (2002b) in particular displays a strong relationship between veteran features and saproxylic presence, further suggesting the use veteran features as signposts for saproxylic species. Veteran features are crucial within this study as they are inherently linked to the age and condition of trees (Hopkins et al., 2005; Fay, 2002; Alexander, 2008b) both of which have been identified important for G. nobilis (Whitehead, 2003; Alexander, 2008b). The observed effectiveness of veteran features as indicators of presence surrounds the associated creation of suitable habitat for saproxylic species.
As previously described the availability of substrate was an essential feature for occupied habitat in south Worcestershire. However, quality, composition and form also appear influential within this study. This is similar to influences on the presence of other saproxylic species (Jacobs et al., 2007). The composition of decay within this study was measured through an assessment of tree successional stage. During the sequence of tree development, heartwood dies and woody material decays (Boddy and Rayner, 1983). The quality and composition of wood material, as perceived by G. nobilis will vary alongside development and decay (Alexander, 2001). Beetles are particularly sensitive to these changes (Araya, 1993; Jukes et al., 2002; Sverdrup-Thygeson, 2001) and within this study there was an increased presence associated with rot found in mid-to-late successional trees; with only completely living trees displaying absence of G. nobilis. Similar studies assessing this relationship have provided comparable results (Rukke, 2000; Hammond et al., 2001; Yee et al., 2006; Ranius et al., 2009b; Brunet and Isacsson, 2009a; Lassauce et al., 2011; Russo et al., 2011).
The influence of tree species within this study was complex and contradictory. Tree species was included in the logistic regression as a factor influencing G. nobilis presence; with Prunus providing particularly significant influence. Previous research also provides contradictions with regards to the presence or absence of tree species associations. Whitehead (2003) alongside Evans and Whitehead (2005) observed obligations to the genus Prunus, with specific species being particularly favourable (e.g. Purple Pershore). This is reinforced by relationships found in other studies with similar saproxylic species (e.g. Lachat et al., 2006; Oleska et al., 2007 Davies et al., 2008; Dubois et al., 2009; Müller and Goßner, 2010). Yet other research regarding G. nobilis supports the latter conclusion with presence observed in the majority of common orchard cultivars (Hurt and Burrough, 2009; Alexander, 2008b; Lush et al., 2009). Several other studies regarding saproxylic species also complement this conclusion suggesting that it is the individual tree characteristics rather than tree species that are dominant in influencing the presence of saproxylic species (Warren and Key, 1991; Ulyshen and Hanula, 2009). As inferred by Alexander and Bower (2011), the production of wood and decay material is affected by the tree species. Therefore the perceived influence of tree species may be as a consequence of indirect influences on substrate characteristics.
At the tree habitat scale many associations found appear to translate to microhabitat characteristics; influencing characteristics at a finer scale. The majority of results display a distinct link to the volume or composition of wood decay within tree habitats. Quantification of relationships has provided novel insights, suggesting that the substrate requirements at habitat scales are of primary importance.
Orchard characteristics
Results from this study indicated no significant difference between inhabited or uninhabited orchards with regards to several characteristics often assumed to influence the presence of saproxylic species. Both size and quantity of trees displayed directional yet, statistically insignificant relationships. Small sites throughout the landscape remained inhabited, thereby accounting for the absence of relationships. Habitat size does not significantly influence the presence of a number of saproxylic species (Irmler et al., 2010); however these species are observed displaying strong dispersal abilities. Other less mobile, saproxylic species within patch-matrix habitats, were generally more often present in larger habitats (Murcia, 1995; Bender et al., 1998; Sadler et al., 2006; Similä et al., 2006; Sahlin and Schroeder, 2010). Results provided in this study therefore contradict contemporary research which suggests somewhat limited dispersal (Whitehead, 2003). Inferring high dispersal ability allows relationships to be effectively explained, with G. nobilis utilising other proximal habitat with the landscape. Such patch and matrix utilisation has facilitated weak relationships between presence and habitat size in other studies of saproxylic species (Debinsky and Holt, 2000). This opposes the common assumption that the species is restricted to traditional orchard (Alexander, 2008b), potentially frequenting external habitats during active periods.
The density of trees is loosely regulated through the utilisation of traditional orchard management (Burrough and Robertson, 2008); nevertheless significant variation was still present across sampled habitats. Contrary to contemporary saproxylic research (e.g. (Hindmarch and Reid, 2001; Lindhe et al., 2005; Johansson et al., 2007; Horák et al. 2012) densely cultivated sites within this study supported populations. Not only does this contradict said research, it also conflicts previous studies, which suggest that dense cultivation prevents G. nobilis colonisation (Burrough and Robertson, 2008). Relationships are explained by generally high incidences of snags and dead limbs within habitats. These successional features exhibit low canopy cover, and thus minimally perturb sunlight penetration and larvae incubation (Vernon and Vannier, 2001; Müller and Brandl, 2009), factors previously associated with density-absence relationships. This indicates a reduced influence of density due cultivated traditional orchards.
The age of orchards was inferred from historical records of orchard land-use; therefore direct quantitative relationships were not present. All sites supporting contemporary G. nobilis populations were located within areas of continuous, historical orchard land-use. Alexander (2008b) too highlighted the importance of temporal persistence in land-use for G. nobilis. Such persistence appears to relate to decay suitability through increasing microhabitat density and diversity with is concomitant with age (Warren and Key, 1991; Regnery et al., 2013) whilst also facilitated in part by traditional management. Larger scale landscape persistence may also influence presence (Hultberg et al., 2010), yet this was not quantified. This may explain the loss of G. nobilis from many regions due to the poor response to rapid habitat modification (Evans and Whitehead, 2005). This may also explain a number of previous relationships, with persistence as well as presence of a habitat characteristics thereby appearing crucial.
Despite the perceived importance of specific traditional management techniques (Alexander, 2008b, PTES 2008b; Lush et al., 2011) there was no relationship between presence and methods of traditional management. Although, sites which were unsuitably managed (both intensive and derelict) did not support G. nobilis populations. Dominance of floral competition (Jansson, 2009) as a result of insufficient canopy management (Horák and Rébl, 2013) may directly slow the growth of trees (Merwin and Stiles, 1994), and thus hinder transition through the successional sequence. This dense orchard flora also negatively impacted beetles through the perturbation of flight and dispersal (Dubois and Vignon, 2008) in turn reducing resource utilisation. The balance of cultivation and low intensity management therefore regulates conditions for G. nobilis.
Landscape scale
Although significant associations have been found with habitat characteristics at the previous scales, the landscape may still provide a broad influence on the distribution and presence at subordinate scales (e.g. Tscharntke et al., 2005). Landscape characteristics may ultimately influence the availability of local habitat (Dauber et al., 2005) and therefore require consideration.
Landscape characteristics
A single significant statistical association was derived from landscape characteristic analysis: Increases in the percentage of orchard land-use within 1km of inhabited sites increased the probability of G. nobilis presence. Other statistical associations provided by landscape variables were insignificant and weakly related. In general, if organisms are not influenced by landscape characteristics then they may exhibit strong dispersal (Kouki et al., 2012; Lizeé et al., 2012). Therefore the absence of multiple significant landscape associations with G. nobilis indicates the potential for dispersal across the landscape. This further supports evidence within this study contradicting the previous dispersal assumptions provided by Whitehead (2003). This observation of greater dispersal for G. nobilis is also supported by Szacki (1999), who showed that the dispersal of small organisms is often underestimated.
The relationship between presence and volume of proximal orchard habitat has a number of implications for G. nobilis. Results infer that connectivity between orchard habitats must be maintained in order for G. nobilis populations to persist. Habitat connectivity is especially important in these heterogeneous landscape matrices due to the scarcity of suitable habitat, with high connectivity affording diversity and abundance (Östman et al., 2001; Chisholm et al., 2011). Several factors are associated with maintaining connectivity within south Worcestershire. Habitat corridors are one such factor. These linear features connect sites across south Worcestershire. In this instance, corridors allowed for the connection of several inhabited orchards, potentially facilitating dispersal of G. nobilis across the landscape and providing the inhabited clusters identified. Further analysis also showed that sites without indicators of G. nobilis were generally isolated from other suitable habitat, and not connected by corridors of suitable habitat; inferring impacts of habitat fragmentation (Tscharntke et al., 2002; Fahrig, 2003).
In many cases it appeared that isolation was a result of low matrix permeability. Landscape permeability (e.g. Kindlmann et al., 2005; Dubois and Vignon, 2008) is a function of the surrounding habitat matrix (Wagner and Fortin, 2005) and is therefore a product of connectivity and fragmentation. Within south Worcestershire sites surrounded by large areas of arable fields appeared to provide a suitable matrix for dispersal and subsequent habitat colonisation. This lead to the clustering of inhabited sites around regions with generally low vegetation height in matrices and suitable orchard habitat. Nevertheless, small sites situated in such ‘open’ landscapes did not support G. nobilis populations. In these instances it appears that increased exposure derived from open landscape matrices facilitates increased negative, climatic influence on orchards (Chen et al., 1995).
An attempt at estimating exposure was completed through analysis of topography and aspect of the surrounding landscape. However, both features were insignificantly different between inhabited and uninhabited sites, with low topographical variation across the entire sample region (±12.21m). Previous research on saproxylic assemblages displayed significant associations with landscape topography (Franc et al., 2007), however topographic variation was far greater; potentially providing observed impacts. Furthermore, the impact of these factors is determined by climatic conditions, with water availability determining their relative influence (Gallardo-Cruz et al., 2009).
Lastly, isolation and loss of connectivity was also induced by the process of urbanisation across the landscape; with urban land-use introducing further opposition to dispersal. Urban areas severely perturb movement of organisms across the landscape (Gustafson and Gardner, 1996; Kindlmann et al., 2005; Ódor et al., 2006; Hedin et al., 2008). Contemporary studies relate the absence of saproxylic species from habitat to urban landscape barriers inhibiting landscape permeability (Bolger et al., 2000; Gibb and Hochuli, 2002; Brunet and Isacsson, 2009b; Bailey et al., 2010). The most significant landscape barrier influencing presence appears to be that of roads. As detailed in Bhattacharya et al. (2003) roads disrupt habitat and prevent the movement of organisms across the landscape. Inhabited and uninhabited sites are proximal in Worcestershire, this juxtaposition appears to be a result of road infrastructure preventing the dispersal and establishment within certain sites.
The surrounding landscape provided a complex of interactions, with several concepts associated with metapopulation dynamics (Levins, 1969) and patch-matrix landscapes appearing dominant. However, firm conclusions surrounding the impact of the landscape are hindered by a lack of ecological and functional knowledge with regards to G. nobilis. Increases in ecological knowledge will facilitate a better understanding of the controls of habitat characteristics across all scales.
Conclusions
This exploratory research has highlighted several important relationships between G. nobilis and habitat characteristics spanning multiple spatial scales. Many of these relationships encompass characteristics previously unconsidered for G. nobilis. Two spatial scales dominated the presence of G. nobilis within south Worcestershire. Whilst uninhabited sites displayed few of the common features found in inhabited orchard habitats, implying that intra-habitat characteristics ultimately determine G. nobilis distribution.
Habitat scale characteristics were particularly influential. Within the microhabitat scale, temperature and humidity were defining factors, providing both direct and indirect influences on G. nobilis. The nature of the decay substrate and microclimates found at this scale appear particularly important, yet these characteristics are particularly hard to replicate through conservation. However, the multi-scalar nature of habitat characteristics allows for the production of desired fine scale characteristics through alterations in broader scales. Tree scale associations were dominated by several characteristics which inferred the quality and quantity of decay habitat within sites. Across the orchard scale no significant relationships were observed. Yet, the intermediate levels of management generally found within inhabited orchards provided suitable habitat for G. nobilis.
At the landscape scale associations exhibited were generally weak. The amount of orchard landscape proximate to sites was influential. However, the nature of their influence is dependent on the dispersal ability of G. nobilis. Information regarding this ability remains vague, therefore the importance of landscape characteristics for G. nobilis could be obscured due to this deficit of knowledge.
This multi-scalar, interrelated influence of habitat characteristics contrasts previous research observing influences derived from relatively discrete factors. However, the relative influence of each scale was significantly different. It therefore is apparent that G. nobilis presence is affected to a greater extent by habitat conditions, as opposed to the landscape composition proximal to sites.
References
Abrahamsson M. and Lindbladh M. (2006) A comparison of saproxylic beetle occurrence between man-made high- and low-stumps of spruce (Picea abies), Forest Ecology and Management, Vol. 226(1), 230-237.
Alder D. and Synott T J. (1992) Permanent sample plot techniques for mixed tropical forest, Tropical Forestry Papers, Vol. 25, 77-80.
Alexander K N A. (1998) The links between forest history and biodiversity: the invertebrate fauna of the ancient pasturewoodlands in Britain and its conservation, in Kirby K J W (ed), The ecological history of European history, Cab international, New York, 73-80.
Alexander K N A. (1999) The invertebrates of Britain’s wood pastures, British Wildlife, Vol. 11(2), 108-117.
Alexander K N A. (2001) What are veteran trees? Where are they found? Why are they important?, in Read H., Forfang A. S., Marciau R., Paltto H., Andersson L. and Tardy B. (eds), Tools for preserving woodland biodiversity, Textbook 2, NACONEX, Sweden, 28-31.
Alexander K N A. (2002) The Noble Chafer Gnorimus nobilis in Worcestershire – a report on the 2002 survey, Unpublished report for The People’s Trust for Endangered Species.
Alexander K N A. (2003) The British saproxylic invertebrate fauna, Proceedings of the second pan – European conference on saproxylic beetles, PTES, London.
Alexander K N A. (2008a) Tree biology and saproxylic coleoptera: issues of definitions and conservation language, Revue d’Ecologie (la Terre et la Vie), Vol. 63, 1-5.
Alexander K N A. (2008b) The Special Importance of Traditional Orchards for Invertebrate conservation, with a Case Study of the BAP Priority Species the Noble Chafer Gnorimus nobilis, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 12-18.
Alexander K N A. (2010) Saproxylic beetles, in Newton A C. (ed.) Biodiversity in the New Forest, Pisces Publications, Newbury, 46-53.
Alexander K N A. and Bower L. (2011) The Noble Chafer and traditional orchards – an old growth species in the English cultural landscape, British Wildlife, Vol. 23(1), 17-22.
Alinvi O., Ball J P., Danell., Hjältén J. and Pettersson R B. (2007) Sampling saproxylic beetle assemblages in dead wood logs: comparing window and eclector traps to traditional bark sieving and refinement, Journal of Insect Conservation, Vol. 11, 99-112.
Araya K. (1993) Relationship between the decay types of dead wood and occurrence of Lucanid beetles, Applied Entomology and Zoology, Vol. 28(1), 27-33.
Atkinson G. and Winnall R A. (2008) Rejuvenating Traditional Orchards, How Multidisciplinary Landscape Partnership Schemes Can Serve as a Vehicle for Restoration – Wyre Forest, West Midlands, United Kingdom, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 19-22.
Bailey D., Schmidt-Entling M H., Eberhart P., Herrmann J D., Hofer G., Kormann U. and Herzog F. (2010) Effects of habitat amount and isolation on biodiversity in fragmented traditional orchards, Journal of Applied Ecology, Vol. 47(5), 1003-1013.
Barker S., Burrough A., Cordrey L., Merry K. and Wedge C. (2011) Conserving the wildlife of traditional orchards, British Wildlife, Vol. 23(1), 8-16.
Bässler C., Müller J., Dziock F. and Brandl R. (2010) Effects of resource availability and climate on the diversity of wood-decaying fungi, Journal of Ecology, Vol. 98, 822-832.
Ben-David M., Blundell G M., Kern J W., Maier J A K., Brown E D. and Jewett S C. (2005) Communication in river otters: Creation of variable resource sheds for terrestrial communities, Ecology, Vol. 86(5), 1331-1345.
Bender D J., Contreras T A. and Fahrig L. (1998) Habitat loss and population decline: a meta-analysis of the patch size effect, Ecology, Vol. 79(2), 517-533.
Benton T G., Bryant D M., Cole L. and Crick H Q P. (2002) Linking agricultural practice to insect and bird populations: a historical study over three decades, Journal of Applied Ecology, Vol. 39(4), 673-687.
Berg Å., Ehnström B., Gustavsson L., Hallingbäck T., Jonsell M. and Weslien J. (1994) Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations, Conservation Biology, Vol. 8, 718-731.
Bergman K-O., Jansson N., Claesson K., Palmer M W. and Milberg P. (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles, Forest Ecology and Management, Vol. 265, 133-141.
Bhattacharya M., Primack R B. and Gerwein J. (2003) Are roads and railroads barriers to bumblebee movement in a temperate urban conservation area, Biological Conservation, Vol. 109, 37-45.
Bignal E M. and McCracken D I. (1996) Low-intensity farming systems in the conservation of the countryside, Journal of Applied Ecology, Vol. 33(3), 413-424.
Billeter R., Liira J., Bailey D., Bugter R., Arens P., Augenstein I., Aviron S., Baudry J., Bukacek R., Burel F., Cerny M., De Blust G., De Cock R., Diekötter T., Dietz H., Dirksen J., Dormann C., Durka W., Frenzel M., Hamersky R., Hendrickx F., Herzog F., Klotz S., Koolstra B., Lausch A., Le Couer D., Maelfait J P., Opdam P., Roubalova M., Schermann A., Schermann N., Schmidt T., Schweiger O., Smulders M J M., Speelsmans M., Simova P., Verboom J., Van Wingerden W K R E., Zobel M. and Edwards P J. (2008) Indicators for biodiversity in agricultural landscapes: a pan-European study, Journal of Applied Ecology, Vol. 45(1), 141-150.
Boddy L. and Rayner A D M. (1983) Origins of decay in living deciduous trees: the role of moisture content and a re-appraisal of the expanded concept of tree decay, New Phytologist, Vol. 94, 623-641.
Bolger D T., Suarez A V., Crookis K R., Morrison S A. and Case T J. (2000) Arthropods in urban habitat fragments in southern California: area, age and edge effects, Ecological Applications, Vol. 10(4), 1230-1248.
Bouchard P., Grebennikov V V., Smith A B T. and Douglas H. (2009) Biodiversity of Coleoptera, in Foottit R G. and Adler P H. (eds.) Insect Biodiversity: Science and Society, Wiley-Blackwell, Oxford, 265-301.
Boyatzis R E. (1998) Transforming Qualitative Information: Thematic Analysis and Code Development, SAGE publications, London.
Brown M W. and Schmitt J J. (2001) Seasonal and diurnal dynamics of beneficial insect populations in apple orchards under different management intensity, Environmental Entomology, Vol. 30(2), 415-424.
Brunet J. and Isacsson G. (2009a) Influence of snag characteristics on saproxylic beetle assemblages in a south Swedish beech forest, Journal of Insect Conservation, Vol. 13, 515-528.
Brunet J. and Isacsson G. (2009b) Restoration of beech forest for saproxylic beetles – effects of habitat fragmentation and substrate density on species diversity and distribution, Biodiversity and Conservation, Vol. 18(9), 2387-2404.
Burrough A. and Robertson H. (2008) Traditional Orchard Survey – Mapping England’s Traditional Orchards, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 26-31.
Buse J., Schröder B. and Assmann T. (2007) Modelling habitat and spatial distribution of an endangered longhorn beetle – A case study for saproxylic insect conservation, Biological Conservation, Vol. 137(3), 372-381.
Buse J., Ranius T. and Assmann T. (2008) An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer, Conservation Biology, Vol. 22(2), 329-337.
Butler J., Alexander K. and Green T. (2002) Decaying Wood: An Overview of Its Status and Ecology in the United Kingdom and Continental Europe, USDA Forest Service General Technical Report PSW-GTR-181, 11-19.
Cardoso P., Aranda S C., Lobo J M., Dinis F., Gaspar C. and Borges P A V. (2009) A spatial scale assessment of habitat effects on arthropod communities of an oceanic island, Acta Oecologica, Vol. 35(5), 590-597.
Chase J M. (2007) Drought mediates the importance of stochastic community assembly, Proceedings of the National Academy of Sciences of the USA, Vol. 104(44), 17430-17434.
Chen J., Franklin J F. and Spies T A. (1995) Growing-season microlcimate gradients from clearcut edges into old-growth Douglas-fir forests, Ecological Applications, Vol. 5(1), 74-86.
Chesmore E D. (2001) Application of time domain signal coding and artificial neural networks to passive acoustical identification of animals, Applied Acoustics, Vol. 62(12), 1359-1374.
Chesmore E. D. and Ohya E. (2004) Automated identification of field-recorded songs of four British grasshoppers using bioacoustic signal recognition, Bulletin of Entomological Research, Vol. 94, 319–330.
Chiari S., Lorenzo M., Audisio P. and Ranius T. (2012) Habitat of an Endangered Saproxylic Beetle, Osmoderma eremita, in Mediterranean Woodlands, Ecoscience, Vol. 19(4), 299-307.
Chisholm C., Lindo Z. and Gonzalez A. (2011) Metacommunity diversity depends on connectivity and patch arrangement in heterogeneous habitat networks, Ecography, Vol. 34(3), 415-424.
Cloke P. and Jones O. (2001) Dwelling, place, and landscape: an orchard in Somerset, Environment and Planning A, Vol. 33, 649-666.
Collinge S K. (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns, Ecology, Vol. 81, 2211–2226.
Cooter J., Dibb J R. and Walsh D B. (1991) A Coleopterists Handbook, Third edition, The Amateur Entomological Society, Feltham.
Cordrey L., Bullock D J., Barker S., Bouch D. and Groves C. (2008) Orchards in The National Trust: an Overview of their History, Economics, Wildlife and People, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 42-50.
Dauber J., Purtauf T., Allspach A., Frisch J., Voigtländer K. and Wolters V. (2005) Local vs. Landscape controls on diversity: a test using surface-dwelling soil macroinvertebrates of differing mobility, Global Ecology and Biogeography, Vol. 14, 213-221.
Davies Z G., Tyler C., Stewart G B. and Pullin A S. (2008) Are current management recommendations for saproxylic invertebrates effective? A systematic review, Biodiversity Conservation, Vol. 17, 209-234.
Debinsky D M. and Holt R D. (2000) A survey and overview of habitat fragmentation experiments, Conservation Biology, Vol. 14, 342–355.
Djupström L B., Perhans K., Weslien J., Schroeder L M., Gustafsson L. and Wikberg S. (2010) Co-variation of lichens, bryophytes, saproxylic beetles and dead wood in Swedish boreal forests, Systematics and Biodiversity, Vol. 8(2), 247-256.
Dominy N J. and Duncan B. (2001) GPS and GIS methods in and African Rain Forest: Applications to tropical ecology and conservation, Conservation Ecology, Vol. 5(2), Article 6.
Duarte F., Jones N. and Fleskens L. (2008) Traditional olive orchards on sloping land: Sustainability or abandonment?, Journal of Environmental Management, Vol. 89(2), 86-98.
Dubois G F. and Vignon V. (2008) First results of radio tracking of Osmoderma eremita (Coleoptera: Cetoniidae) in French chestnut orchards, Revue d’Ecologie, Terre et Vie, Vol. 10, 131-138.
Dubois G F., Vignon V., Delettre Y R., Rantier., Vernon P. and Burel F. (2009) Factors affecting the occurrence of the endangered saproxylic beetle Osmoderma eremita (Scopoli, 1763) (Coleoptera: Certoniidae) in an agricultural landscape, Landscape and Urban Planning, Vol. 91, 152-159.
Elith J. and Leathwick J R. (2009) Species Distribution Models: ecological explanation and prediction across space and time, The Annual Review of Ecology, Evolution and Systematics, Vol. 40, 677-697.
Epstein D L., Zack R S., Brunner J F., Gut L. and Brown J J. (2001) Ground Beetle Activity in Apple Orchards under reduced pesticide management regimes, Biological Control, Vol.21(2), 97-104.
Evans H C. and Whitehead P F. (2005) Entomogenous fungi of arboreal Coleoptera from Worcestershire, England, including the new species Harposporium bredonense. Mycological Progress, Vol. 4(2), 91-99.
Ewers R M., Thorpe S. and Didham R K. (2007) Synergistic interactions between edge and area effects in a heavily fragmented landscape, Ecology, Vol. 88(1), 96-106.
Fahrig L. (2003) Effects of habitat fragmentation on Biodiversity, Annual Review of Ecology, Evolution, and Systematics, Vol. 34, 487-515.
Farr I. and Chesmore D. (2007) Automated bioacoustic detection and identification of wood-boring insects for quarantine screening and insect ecology, Proceedings of the 4th International Conference on Bioacoustics, Vol. 29(3), 201–208.
Fay N. (2002) Environmental arboriculture, tree ecology and veteran tree management, Arboricultural Journal, Vol. 26(3), 213-238.
Fetridge E D., Ascher J S., Langellotto G A. (2008) The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea), Annals of the Entomological Society of America, Vol. 101(6), 1067-1077.
Fierke M K., Kinney D L., Salisbury V B., Crook D J. and Stephen F M. (2005) Development and comparison of intensive and extensive sampling methods and preliminary within-tree population estimates of Red Oak Borer (Coleoptera: Cerambycidae) in the Ozark Mountains of Arkansas, Environmental Entomology, Vol. 34(1), 184-192.
Franc N., Götmark F., Økland B., Nordén B. and Paltto H. (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest, Biological Conservation, Vol. 135, 86-98.
Frank D., Finckh M. and Wirth C. (2009) Impacts of land use on habitat functions of old-growth forests and their biodiversity, in Wirth C., Gleixner G. and Heimann M. (eds.) Old Growth Forests: Function, Fate and Value, Ecological Studies, Vol. 207, Springer, Berlin.
Franklin J F., Spies T A., Van Pelt R., Carey A B., Thornburgh D A., Berg D R., Lindenmayer D B., Harmon M E., Leeton W S., Shaw D C., Bible K. and Chen J. (2002) Disturbance and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir as an example, Forest Ecology and Management, Vol. 155(1), 399-423.
Gallardo-Cruz J A., Pérez-García E A. and Meave J A. (2009) β-diversity and vegetation structure as influenced by slope aspect and altitude in a seasonally dry tropical landscape, Landscape Ecology, Vol. 24(4), 473-482.
García Torres L., Peña-Barragán J M., López-Granados., Jurado-Expósito M. and Fernández-Escobar R. (2008) Automatic assessment of agro-environmental indicators from remotely sensed images of tree orchards and its evaluation using olive plantations, Computers and Electronics in Agriculture, Vol. 61(2), 179-191.
Gibb H. and Hochuli D F. (2002) Habitat fragmentation in an urban environment: large and small fragments support different arthropod assemblages, Biological Conservation, Vol. 106(1), 91-100.
Gibb H., Pettersson R B., Hjältén J., Ball J P., Johansson T., Atlegrim O. and Danell K. (2006) Conservation-orientated forestry and early successional saproxylic beetles: Responses of functional groups to manipulated dead wood substrates, Biological Conservation, Vol. 129(4), 437-450.
Groombridge B. (1992) Global biodiversity: status of the earth’s living resources, Chapman and Hall, London.
Grove S J. (2002a) Saproxylic insect ecology and the sustainable management of forests, Annual Review of Ecology and Systematics, Vol. 33, 1-23.
Grove S J. (2002b) Tree basal are and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics, Ecological Indicators, Vol. 1(3), 171-188.
Grove S J. (2002c) Tree basal area and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics, Ecological Indicators, Vol. 1(3), 171-188.
Grove S J. (2002d) The influence of forest management history on the integrity of the saproxylic beetle fauna in an Australian lowland tropical rainforest, Biological Conservation, Vol. 104(2), 149-171.
Gustafson E J. and Gardner R H. (1996) The effect of landscape heterogeneity on the probability of patch colonisation, Ecology, Vol. 77(1), 94-107.
Hammond H J., Langor D W. and Spence J R. (2001) Early colonization of Populus wood by saproxylic beetles (Coleoptera), Canadian Journal of Forest Research, Vol. 31(7), 1175-1183.
Hammond H J., Langor D W. and Spence J R. (2004) Saproxylic beetles (Coleoptera) using Populus in boreal aspen stand of western Canada: spatiotemporal variation and conservation of assemblages, Canadian Journal of Forest Research, Vol. 34(1), 1-19.
Hannah L., Carr J L. and Lankerani A. (1995) Human disturbance and natural habitat: a biome level analysis of a global dataset, Biodiversity Conservation, Vol. 2, 128-155.
Hedgren O. and Weslien J. (2008) Detecting rare species with random or subjective sampling: a case study of red-listed saproxylic beetles in boreal Sweden, Conservation Biology, Vol. 22(1), 212-215.
Hedin J., Ranius T., Nilsson S G. and Smith H G. (2008) Restricted dispersal in a flying beetle assessed by telemetry, Biodiversity and Conservation, Vol. 17(3), 675-684.
Herzog F. (1998) Streuobst: a traditional agroforestry system as a model for agroforestry development in temperate Europe, Agroforestry Systems, Vol. 42(1), 61-80.
Hindmarch T D. and Reid M L. (2001) Thinning of mature lodgepole pine stands increases scolytid bark beetle abundance and diversity, Canadian Journal of Forest Research, Vol. 31(9), 1502-1512.
Hjätlén J., Johansson T., Alinvi O., Danell K., Ball J P., Petterson R., Gibb H. and Hilszczański J. (2007) The importance of substrate type, shading and scorching for the attractiveness of dead wood to saproxylic beetles, Basic and Applied Ecology, Vol. 8(2), 364-376.
Holland J D., Bert D G. and Fahrig L. (2004) Determining the spatial scale of species’ response to habitat, BioScience, Vol. 54(3), 227-233.
Holland J D., Fahrig L. and Cappuccino N. (2005) Body size affects the spatial scale of habitat-beetle interactions, Oikos, Vol. 110, 101-108.
Hopkins A J M., Harrison K S., Grove S J., Wardlaw T J. and Mohammed C L. (2005) Wood-decay fungi and saproxylic beetles associated with living Eucalyptus obliqua trees: early results from studies at the Warra LTER Site, Tasmania, Tasforests, Vol. 16, 111- 126.
Hopkins J J. and Kirby K J. (2007) Ecological change in British broadleaved woodland since 1947, Ibis, Vol. 149(s2), 29-40.
Horák J., Vávrová E. and Chobot K. (2010) Habitat preferences influencing populations, distribution and conservation of the endangered saproxylic beetle Cucujus cinnaberinus (Coleoptera: Cucujidae) at the landscape level, European Journal for Entomology, Vol. 107(1), 81-88.
Horák J., Chumanová E. and Hilszański J. (2012) Saproxylic beetle thrives on the openness in management: a case study on the ecological requirements of Cucujus cinnaberinus from Central Europe, Insect Conservation and Diversity, Vol. 5(6), 403-413.
Horák J. and Rébl K. (2013) The species richness of click beetles in ancient pasture benefits from a high level of sun exposure, Journal of Insect Conservation, Vol. 17, 307-318.
Hottola J. and Siitonen J. (2008) Significance of woodland key habitats for polypore diversity and red-listed species in boreal forests, Biodiversity and Conservation, Vol. 17(11), 2559-2557.
Hultberg T., Brunet J., Broström A. and Lindbladh M. (2010) Forest in a cultural landscape – the vegetation history of Torup in southernmost Sweden, Ecological Bulletins, Vol. 53, 141-153.
Hurt L. and Burrough A. (2009) Nobel Chafer survey 2009, Brockhampton Estate, Herefordshire, PTES, London.
Irmler U., Arp H. and Nötzold R. (2010) Species richness of saproxylic beeltes in woodlands affected by dispersion ability of species, age and stand size, Journal of Insect Ecology, Vol. 14, 227-235.
Jacobs J M., Spence J R. and Langor D W. (2007) Influence of boreal forest succession and dead wood qualities on saproxylic beetles, Agricultural and Forest Entomology, Vol. 9(1), 3-16.
Jansson N. (2009) Habitat requirements and preservation of the beetle assemblages associated with hollow oaks, Doctoral Thesis: Department of Physics, Chemistry and Biology, Linköping University, Linköping, Sweden.
Jansson N., Ranius T., Larsson A. and Milberg P. (2009) Boxes mimicking tree hollows can help conservation of saproxylic beetles, Biodiversity Conservation, Vol. 18(14), 3891-3908.
Jeanneret Ph., Schüpbach B. and Luka H. (2003) Quantifying the impact of landscape and habitat features on biodiveristy in cultivated landscapes, Agriculture, Ecosystems and Environment, Vol. 98, 311-320.
Johansson T., Hjältén J., Gibb H., Hilszczanski J., Stenlid J., Ball J P., Alinvi O. and Danell K. (2007) Variable response of different functional groups of saproxylic beetles to substrate manipulation and forest management: Implications for conservation strategies, Forest Ecology and Management, Vol. 242(2), 496-510.
Joint Nature Conservation Committee (2007) Conserving Biodiversity – the UK Approach, JNCC, Peterborough.
Joint Nature Conservation Committee (2010) UK priority species pages – version 2 – Gnorimus nobilis, JNCC, Peterborough.
Jonsell M., Weslien J. and Ehnström B. (1998) Substrate requirements of red-listed saproxylic invertebrates in Sweden, Biodiversity and Conservation, Vol. 7, 749-764.
Jonsell M., Nordlander G. and Ehnström B. (2001) Substrate associations of insects breeding in fruiting bodies of wood-decaying fungi, Ecological Bulletins, Vol. 49, 173-194.
Jonsell M. and Nordlander G. (2002) Insects in polypore fungi as indicator species: a comparison between forest sites differing in amounts and continuity of dead wood, Forest Ecology and Management, Vol. 157(1), 101-118.
Jonsell M. and Weslien J. (2003) Felled or standing retained wood – it makes a difference for saproxylic beetles, Forest Ecology and Management, Vol. 175(1-3), 425-435.
Jonsell M., Schroeder M. and Weslien J. (2005) Saproxylic beetles in high stumps of spruce: Fungal flora important for determining the species composition, Scandinavian Journal of Forest Research, Vol. 20(1), 54-62.
Jonsson B G., Kruys N. And Ranius T. (2005) Ecology of species living on dead wood – lessons for dead wood management, Silva Fennica, Vol. 39(2), 289-309.
Jørgen K. and Karsten T. (1994) A new method for measuring tree height in tropical rain forest, Journal of Vegetation Science, Vol. 5, 139-140.
Jukes M R., Ferris R. and Peace A J. (2002) The influence of stand structure and composition on diversity of canopy Coleoptera in coniferous plantations in Britain, Forest Ecology and Management, Vol. 163(1-3), 27-41.
Kaila L., Martikainen P., Punttila P. and Yakovlev E. (1994) Saproxylic beetles (Coleoptera) on dead birch trunks decayed by different polypore species, Annals of Zoology Fennici, Vol. 31, 97-107.
Kindlmann P., Aviron S. and Burel F. (2005) When is landscape matrix important for determining animal fluxes between resource patches?, Ecological Complexity 2, 150-158.
Kirby K J., Thomas R C., Key R S., McLean I F G. and Hodgetts N. (1995) Pasture-woodland and its conservation in Britain, Biological Journal of the Linnean Society, Vol. 56(s1), 135-153.
Kouki J., Hyvärinen E., Lappalainen H., Martikainen P. and Simila M. (2012) Landscape context affects the success of habitat restoration: large-scale colonization patterns of saproxylic and fire-associated species in boreal forests, Diversity and Distributions, Vol. 18, 348-355.
Koziell S P. (2000) A tale of two apples, Resurgence, Vol. 198, 44-45.
Krikken J. (1984) A new key to the suprageneric taxa in the beetle family Cetoniidae, with annotated lists of the known genera, Zoologische Verhandelingen, Vol. 210(1), 1-75.
Lachat T., Nagel P., Cakpo Y., Attignon S., Goergen G., Sinsin B. and Peveling R. (2006) Dead wood and saproxylic beetle assemblages in a semi-deciduous forest in Southern Benin, Forest and Ecology Management, Vol. 225(1-3), 27-38.
Lachat T., Wermelinger B., Gossner M M., Buβler H., Isacsson G. and Müller J. (2012) Saproxylic beetles as indicator species foe dead-wood amount and temperature in European beech forests, Ecological Indicators, Vol. 23, 323-331.
Lang G A. (2001) Intensive Sweet Cherry Orchard Systems – Rootstocks, Vigor, Precocity, Productivity and Management, The Compact Fruit Tree, Vol. 34(1), 23-26.
Larrieu L., Cabanettes A. and Delarue A. (2012) Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees, European Journal of Forest Research, Vol. 131(3), 773-786.
Lassauce A., Paillet Y., Jactel H. and Bouget C. (2011) Deadwood as a surrogate for forest biodivsersity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms, Ecological Indicators, Vol. 11(5), 1027-1039.
Legendre P. (1993) Spatial autocorrelation: Trouble or New Paradigm?, Ecology, Vol. 74(6), 1659-1673.
Levins R. (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control, Bulletin of the Entomological Society of America, Vol. 15, 237-240.
Lindhe A., Lindelöw A. and Åsenblad N. (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter, Biodiversity and Conservation, Vol. 14, 3033-3053.
Lindo Z. Winchester N N. and Didham R K. (2008) Nester patterns of community assembly in the colonisation of artificial canopy habitats by oribatid mites, Oikos, Vol. 117(12), 1856-1864.
Lindo Z. and Winchester N N. (2009) Spatial and environmental factors contributing to patterns in arboreal and terrestrial oribatid mite diversity across spatial scales, Oecologia, Vol. 160(4), 817-825.
Lizeé M-H., Manel S., Mauffrey J-F., Tatoni T. and Deschamps-Cottin M. (2012) Matrix configuration and patch isolation influences override the species-area relationship for urban butterfly communities, Landscape Ecology, Vol. 27(2), 159-169.
Löfman S. and Kouki J. (2001) Fifty years of landscape transformation in managed forests of southern Finland, Scandinavian Journal of Forest Research, Vol. 16(1), 44-53.
Lush M., Robertson H J., Alexander K N A., Giavarni V., Hewins E., Mellings J., Stevenson C R., Storey M. and Whitehead P F. (2009) Biodiversity studies of six traditional orchards in England, Natural England Research Reports, No. 025.
MacDonald D W., King C M. and Strachan R. (2007) Introduced species and the line between biodiversity conservation and naturalistic eugenics, in Macdonald D. and Service K. (eds.), Key Topics in Conservation Biology, Blackwell, Malden, 186-205.
Mackey B G. and Lindenmayer D B. (2001) Towards a hierarchical framework for modelling the spatial distribution of animals, Journal of Biogeography, Vol. 28(9), 1147-1166.
Mäkipää R. and Linkosalo T. (2011) A non-destructive field method for measuring wood density of decaying logs, Silva Fennica, Vol. 45(5). 1135-1142.
Mankin R W., Mizrach A., Hetzroni A., Levsky S., Nakache Y. and Soroker V. (2008) Temporal and spectral features of sounds of wood-boring beetle larvae: identifiable patterns of activity enable improved discrimination from background noise, Florida Entomologist, Vol. 91(2), 241-248.
Marshall D. (2008) Herefordshire orchard community evaluation project, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 96-100.
Martikainen P., Siitonen J., Punttila P., Kaila L. and Rauh J. (2000) Species richness of Coleoptera in mature managed and old-growth boreal forests in southern Finland, Biological Conservation, Vol. 94(2), 199-209.
Martikainen P. (2001) Conservation of threatened saproxylic beetles: significance of retained aspen Populus tremula on clearcut areas, Ecological Bulletins, Vol. 49, 205-218.
Martikainen P. and Kouki J. (2002) Sampling the rarest: threatened beetles in boreal forest biodiversity inventories, Biodiversity and Conservation, Vol. 12(9), 1815-1831.
Mattson K G., Swank W T. and Waide J B. (1987) Decomposition of woody debris in a regenerating, clear-cut forest in the Southern Appalachians, Canadian Journal of Forest Research, Vol. 17(7), 712-721.
Mazzerolle M J. and Villard M. (1999) Patch characteristics and landscape context as predictors of species presence and abundance: a review, Ecoscience, Vol. 6(1), 117-124.
McGeoch M A., Schroeder M., Ekbom B. and Larsson S. (2007) Saproxylic beetle diversity in a managed boreal forest: importance of stand characteristics and forestry conservation measures, Diversity and Distributions, Vol. 13(4), 418-429.
Meggs J M., Munks S A. and Corkrey R. (2003) The distribution and habitat characteristics of a threatened lucanid beetle Hoplogonus simsoni in north-east Tasmania, Pacific Conservation Biology, Vol. 9(3), 172-186.
Merwin I A. and Stiles W C. (1994) Orchard groundcover management impacts on apple tree growth and yield, and nutrient availability and uptake, Journal of the American Society for Horticultural Science, Vol. 199(2), 209-215.
Michel A K. and Winter S. (2009) Tree microhabitat structures as indicators of biodiversity in Douglas-fir forests of different stand ages and management histories in the Pacific Northwest, U.S.A., Forest Ecology and Management, Vol. 257(6), 1453-1464.
Molina-Montenegro M A., Badano E I. and Cavieres L A. (2006) Cushion plants as microclimatic shelters for two ladybird species in alpine zone of central Chile, Arctic, Antarctic and Alpine Research, Vol. 38(2), 224-227.
Müller J., Buβler H. And Kneib T. (2008) Saproxylic beetle assemblages related to silvicultural management intensity and stand structures in a beech forest in Southern Germany, Journal of Insect Conservation, Vol. 12, 107-124.
Müller J. and Brandl R. (2009) Assessing biodiversity by remote sensing in mountainous terrain: the potential of LiDAR to predict forest beetle assemblages, Journal of Applied Ecology, Vol. 46(4), 897-905.
Müller J. and Bütler R. (2010) A review of habitat thresholds for dead wood: a baseline for management recommendations in European forests, European Journal of Forest Research, Vol. 129(6), 981-992.
Müller J. and Goβner M M. (2010) Three-dimensional partitioning of diversity informs state-wide strategies for the conservation of saproxylic beetles, Biological Conservation, Vol. 143(3), 625-633.
Murcia C. (1995) Edge effects in fragmented forests: implications for conservation, Trends in Ecology and Evolution, Vol. 10(2), 58-62.
Murphy H T. and Lovett-Doust J. (2004) Context and connectivity in plant metapopulations and landscape mosaics: does the matrix matter?, Oikos, Vol. 105(1), 3-14.
Nagendra H., Lucas R., Pradinho Honrado J., Jongman R H G., Tarantino C., Adamo M. and Mairota P. (2013) Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat conditions, species diveristy and threats, Ecological Indicators, Vol. 33(1), 45-59.
Natural England (2012) Nature on the Map, http://www.natureonthemap.naturalengland .org.uk/map.aspx?m=bap, accessed: 23/03/2013.
Niemelä J. (1997) Invertebrates and Boreal forest management, Conservation Biology, Vol. 11(3), 601-610.
Nieto A. and Alexander K N A. (2010) European Red List of Saproxylic Beetles, Publication Office of the EU, Luxembourg.
Nilsson S G. (1997) Forests in the temperate-boreal transition: natural and man-made features, Ecological Bulletins, Vol. 46, 61-71.
Nilsson S G. and Baranowski R. (1997) Habitat predictability and the occurrence of wood beetles in old-growth beech forests, Ecography, Vol. 20, 491-498.
Nilsson S G., Hedin J. and Niklasson M. (2001) Biodiversity and its assessment in boreal and nemoral forests, Scandinavian Journal of Forest Research, Vol. 16 (s3), 10-26.
Nilsson S G., Niklasson M., Hedin J., Aronsson G., Gutowski J M., Linder P., Ljungberg H., Mikusinski G., Ranius T. (2002) Densities of large living and dead trees in old-growth temperate and boreal forests, Forest Ecology and Management, Vol. 161(1), 189-204.
Ódor P., Heilmann-Clausen J., Christensen M., Aude E., van Dort K W., Piltaver A., Siller I., Veerkamp M T., Walleyn R., Standovár T., van Hees A F M., Kosec J., Matočec N., Kraigher H. and Grebenc T. (2006) Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe, Biological Conservation, Vol. 131(1), 58-71.
Økland B., Bakke A., Hågvar S. And Kvamme T. (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway, Biodiversity and Conservation, Vol. 5, 75-100.
Oleska A., Urlich W. and Gawroński R. (2007) Host tree preferences of Hermit beetles (Osmoderma eremit Scop., Coleoptera: Scarabaeidae) in a network of rural avenues in Poland, Polish Journal of Ecology, Vol. 55(2), 315-323.
Orłowski G. and Nowak L. (2007) The importance of marginal habitats for the conservation of old trees in agricultural landscapes, Landscape and Urban Planning, Vol. 79(1), 77-83.
Osborne P J. (1974) An insect assemblage of early Flandrian age from Lea Marston, Warwickshire, and its bearing on the contemporary climate and ecology, Quaternary Research, Vol. 4(4), 471-486.
Östman Ö., Ekbom B. and Bengtsson J. (2001) Landscape heterogeneity and farming practice influence biological control, Basic and Applied Ecology, Vol. 2(4), 365-371.
Paracchini M L., Terres J M., Petersen J E. and Hoogeveen Y B. (2007) High nature value farmland and traditional agricultural landscapes, in Pedroli B., Van Doorn A., De Blust G., Paracchini M L., Wascher D. and Bunce F. (eds.), Europe’s living landscapes. Essays on exploring our identity in the countryside, Landscape Europe, Wageningen, 22-34.
Pentillä R., Siitonen J. and Kuusinen M. (2004) Polypore diversity in managed and old growth boreal Picea abies forests in southern Finland, Biological Conservation, Vol. 117(3), 271-283.
People’s Trust for Endangered Species (2008a) Noble Chafer Fact sheet, PTES, London.
People’s Trust for Endangered Species (2008b) Noble Chafer (Gnorimus nobilis), PTES, London.
Petit C C. and Lambin E F. (2002) Impact of data integration technique on historical land-use/land-cover change: Comparing historical maps with remote sensing data in the Belgian Ardennes, Landscape Ecology, Vol. 17(2), 117-132.
Philpott S M., Arendt W J., Armbrecht I., Bichier P., Diestch T V., Gordon C., Greenberg R., Perfecto I., Reynoso-Santos R., Soto-Pinto L., Tejeda-Cruz C., Williams-Linera G., Valenzuela J. and Zolotoff J M. (2008) Biodiversity Loss in Latin American Coffee Landscapes: Review of the Evidence on Ants, Birds, and Trees, Conservation Biology, Vol. 22(5), 1093-1105.
Pimm S L., Russell G J., Gittleman J L. and Brooks T M. (1995) The Future of Biodiversity, Science, Vol. 269(5222), 347-350.
Plieninger T., Höchtl F. and Spek T. (2006) Traditional land-use and nature conservation in European rural landscapes, Environmental Science and Policy, Vol. 9(4), 317-321.
Pyle C. and Brown M M. (1999) Heterogeneity of wood decay classes within hardwood logs, Forest Ecology and Management, Vol. 114(2-3), 253-259.
Ranius T. and Nilsson S G. (1997) Habitat of Osmoderma eremita Scop. (Coleoptera: Scarabaeidae), a beetle living in hollow trees, Journal of Insect Conservation, Vol. 1(4), 193-204.
Ranius T. (2000) Minimum viable metapopulation size of a beetle, Osmoderma eremita, living in tree hollows, Animal Conservation, Vol. 391), 37-43.
Ranius T. and Jansson N. (2000) The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associate with old oaks, Biological Conservation, Vol. 95(1), 85-94.
Ranius T. and Wilander P. (2000) Occurrence of Larca lata H.J. Hansen (Pseudoscorpionida: Garypidae) and Allochernes wideri C.L. Koch (Pseudoscorpionida: Chernetidae) in tree hollows in relation to habitat quality and density, Journal of Insect Conservation, Vol. 4, 23-31.
Ranius T. (2001) Constancy and asynchrony of Osmoderma eremita populations in tree hollows, Oecologia, Vol. 126(2), 208-215.
Ranius T. (2002a) Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden, Biological Conservation, Vol. 103, 85-91.
Ranius T. (2002b) Osmoderma eremita as an indicator of species richness of beetles in tree hollows, Biodiversity and Conservation, Vol. 11(5), 931-941.
Ranius T. and Jannson N. (2002) A comparison of three methods to survey saproxylic beetles in hollow oaks, Biodiversity and Conservation, Vol. 11(10), 1759-1771.
Ranius T. (2003) Habitat fragmentation affects beetles and pseudoscorpions living in hollow oaks in Sweden, Proceedings of the second pan-European conference on Saproxylic Beetles, PTES, London.
Ranius T. (2006) Measuring the dispersal of saproxylic insects: a key characteristics of their conservation, Population Ecology, Vol. 48, 177-188.
Ranius T. and Fahrig L. (2006) Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds, Scandinavian Journal of Forest Research, Vol. 21(3), 201-208.
Ranius T. (2007) Extinction risks in metapopulations of a beetle inhabiting hollow trees predicted from time series, Ecography, Vol. 30(5), 716-726.
Ranius T., Niklasson M. and Berg N. (2009a) Development of tree hollows in pedunculate oak (Quercus robur), Forest Ecology and Management< Vol. 257, 303-310.
Ranius T., Svensson G P., Berg N., Niklasson M. and Larsson M C. (2009b) The successional change of hollow oaks affects their suitability for an inhabiting beetle, Osmoderma eremita, Annals of Zoology Fennici, Vol. 46(3), 205-216.
Ranius T. and Roberge J M. (2011) Effects of intensified forestry on the landscape-scale extinction risk of dead wood dependent species, Biodiversity Conservation, Vol. 20(13), 2867-2882.
Read H. (2000) Veteran trees: A guide to good management, English Nature, Peterborough.
Regnery B., Paillet Y., Couvet D. and Kerbiriou C. (2013) Which factors influence the occurrence and density of tree microhabitats in Mediterranean oak forests?, Forest and Ecology Management, Vol. 295, 118-125.
Renault D., Vernon P. and Vannier G. (2005) Critical thermal maximum and body water loss in first instar larvae of three Cetoniidae species (Coleoptera), Journal of Thermal Biology, Vol. 30(8), 611-617.
Robertson H J. and Wedge C. (2008) Traditional Orchards and the UK Biodiversity Action Plan, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 109-118.
Robinson G M. (1983) The evolution of the horticultural industry in the Vale of Evesham, Scottish Geographical Magazine, Vol. 99(2), 89-100.
Robinson R. and Sutherland W (2002) Post-war changes in arable farming and biodiversity in Great Britain, Journal of Applied Ecology, Vol. 39, 157–176.
Rotheray E L. (2013) Differences in ecomorphology and microhabitat use of four saproxylic larvae (Diptera, Syrphidae) in Scots pine stump rot holes, Ecological Entomology, Vol. 38(3), 219-229.
Rounsevell M D A., Annetts J E., Audsley E., Mayr T. and Reginster I. (2003) Modelling the spatial distribution of agricultural land use at the regional scale, Agriculture, Ecosystems and Environment, Vol. 95., 465-479.
Rukke B A. and Midtgaard F. (1998) The importance of scale and spatial variables for the fungivorous beetle Bolitophagus reticulatus (Coleoptera, Tenebrionidae) in a fragmented forest landscape, Ecography, Vol. 21(6), 561-572.
Rukke B A. (2000) Effects of habitat fragmentation: increased isolation and reduced habitat size reduces the incidence of dead wood fungi beetles in a fragmented forest landscape, Ecography, Vol. 23(4), 492-502.
Russo D., Cistrone L. and Garonna A P. (2011) Habitat selection by the highly endangered longhorn beetle Rosalia alpine in Southern Europe: a multiple spatial scale assessment, Journal of Insect Conservation, Vol. 15(5), 685-693.
Sadler J P., Small E C., Fiszpan H., Teffer M G. and Niemelä (2006) Investigating environmental variation and landscape characteristics of an urban-rural gradient using woodland carabid assemblages, Journal of Biogeography, Vol. 33(6), 1126-1138.
Sahlin E. and Schroeder L M. (2010) Importance of habitat patch size for occupancy and density of aspen-associated saproxylic beetles, Biodiversity Conservation, Vol. 19(5), 1325-1339.
Schiegg K. (2001) Saproxylic insect diversity of beech: limbs are richer than trunks, Forest Ecology and Management, Vol. 149(1-3), 295-304.
Schroeder L M., Ranius T., Ekbom M. and Larsson S. (2007) Spatial occurrence of a habitat-tracking saproxylic beetle inhabiting a managed forest landscape, Ecological Applications, Vol. 17(3), 900-909.
Schweiger O., Maelfait J P., Van Wingerden W K R E., Hendrickx F., Billeter R., Speelmans M., Augenstein I., Aukema B., Aviron S., Bailey D., Bukacek R., Burel F., Diekötter T., Dirksen J., Frenzel M., Herzog F., Liira J., Roubalova M. and Bugter R. (2005) Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales, Journal of Applied Ecology, Vol. 42(6), 1129-1139.
Sebek P., Altman J., Platek M. and Cizek L. (2013) Is active management the key to the conservation of saproxylic Biodiversity? Pollarding promotes the formation of tree hollows, PloS one, Vol. 8(3), e60456, 1-6.
Sharma A. and Panigrahy S. (2007) Apple orchard characterization using remote sensing and GIS in Shimla district of Himachal Pradesh, The Proceedings of the Remote Sensing and Photogrammetry Annual Conference, RSPS, Newcastle,
Sharples L. (2007) Apples, cider and celebration, in Hall C M. and Sharples L. (eds.), Food and wine festivals and events around the world, Butterworth-Heinemann, Oxford, 133-145.
Siitonen J. (1994) Decaying wood and saproxylic Coleoptera in two old spruce forests: a comparison based on two sampling methods, Annals of Zoology Fennici, Vol. 31, 89-95.
Siitonen J. (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example, Ecological Bulletins, Vol. 49, 11-41.
Siitonen J. and Saaristo L. (2000) Habitat requirements and conservation of Pytho kolwensis, a beetle species of old-growth boreal forest, Biological Conservation, Vol. 94(2), 211-220.
Similä M., Kouki J., Mönkkönen M. and Sippola A L. (2002) Beetle species richness along the forest productivity gradient in northern Finland, Ecography, Vol. 25(1), 42-52.
Similä M., Kouki J. and Martikainen P. (2003) Saproxylic beetles in managed and seminatural Scots pine forests: quality of dead wood matters, Forest Ecology and Management, Vol. 174(1-3), 365-381.
Similä M., Kouki J., Mönkkönen M. and Sippola A L. and Huhta E. (2006) Co-variation and indicators of species diversity: Can richness of forest-dwelling species be predicted in northern boreal forests?, Ecological Indicators, Vol. 6(4), 686-700.
Sippola A L., Siitonen J. and Punttila P. (2002) Beetle diversity in timberline forests: a comparison between old-growth and regeneration areas in Finnish Lapland, Annals of Zoology Fennici, Vol. 39, 69-86.
Sirami C., Jay-Robert P., Brustel H., Valladares L., Le Guilloux S., Martin J L., (2008) Saproxylic beetle assemblages of old Holm-oak trees in the Mediterranean region: role of a keystone structure in a changing heterogeneous landscape, Revue d Ecologie (la Terre et La Vie), Vol. 63, 93–106.
Smith M N. (2003) Saproxylic beetles in Britain, an overview of the status and distribution of four Biodiversity Action Plan species, Proceedings of the second pan-European conference on Saproxylic Beetles, PTES, London.
Speight M C D. (1989) Saproxylic invertebrates and their conservation, Council of Europe, Strasbourg.
Stoate C., Báldi A., Beja P., Boatman N D., Herzon I., van Doorn A., de Snoo G R., Rakosy L. and Ramwell C. (2009) Ecological impacts of early 21st century agricultural change in Europe – A review, Journal of Environmental Management, Vol. 91(1), 22-46.
Stoner K J L. and Joern A. (2004) Landscape vs. Local habitat scale influences to insect communities from tallgrass prairie remnants, Ecological Applications, Vol. 14(5), 1306-1320.
Sverdrup-Thygeson A. (2001) Can ‘continuity indicator species’ predict species richness or red-listed species of saproxylic beetles?, Biodiversity and Conservation, Vol. 10, 815-832.
Szacki J. (1999) Spatially structured populations: how much do they match the classic metapopulation concept?, Landscape Ecology, Vol. 14(4), 369-379.
Tahvanainen J O. (1972) Phenology and microhabitat selection of some flea beetles (Coleoptera: Chrysomelidae) on wild and cultivated crucifers in central New York, Insect Systematics and Evolution, Vol. 3(2), 120-138.
Tauzin P. (2000) Le genre Aleurostictus Kirby, 1827. Contribution à sa connaisance et précision sur la distribution des espèces (Coleoptera, Cetoniidae, Trichiinae, Trichiini), L’Entomologiste, Vol. 56(6), 231-281.
Tischendorf L. and Fahrig L. (2000) On the use and measurement of landscape connectivity, Oikos, Vol. 90(1), 7-19.
Tikkanen O-P., Martikainen P., Hyvärinen E., Junninen K. and Kouki J. (2006) Red-listed boreal forest species of Finland: associations with forest structure, tree species, and decaying wood, Annals of Zoology Fennici, Vol. 43, 373-383.
Tikkanen O-P., Heinone T., Kouki J. and Matero K. (2007) Habitat suitability models for saproxylic red-listed boreal forest species in long-term matrix management: Cost-efective measures for multi-species conservation, Biological Conservation, Vol. 140(3-4), 359-372.
Timmer L W., Sandler H A., Graham J H. and Zitko S E. (1988) Sampling citrus orchards in Florida to estimate populations of Phytophthora parasitica, Phytopathology, Vol. 78(7), 940-944.
Tscharntke T., Steffan-Dewenter I., Kruess A. and Thies C. (2002) Characteristics of insect populations on habitat fragments: A mini review, Ecological Research, Vol. 17(2), 229-239.
Tscharntke T., Klein A M., Kruess A., Steffan-Dewenter I. and Thies C. (2005) Landscape perspectives on agricultural intensification and biodiversity – ecosystem service management, Ecology Letters, Vol. 8(8), 857-874.
Turner W., Spector S., Gardiner N., Fladeland M., Sterling E. and Steininger M. (2003) Remote sensing for biodiversity science and conservation, Trends in Ecology and Evolution, Vol. 18(6), 306-314.
Ulyshen M D. and Hanula J L. (2009) Habitat associations of saproxylic beetles in the southeastern United States: A comparison of forest types, tree species and wood postures, Forest Ecology and Management, Vol. 257(2), 653-664.
Vernon P. and Vannier G. (2001) Freezing susceptibility and freezing tolerance in Palaeartic Cetoniidae (Coleoptera), Canadian Journal of Zoology, Vol. 79(1), 67-74.
Wagner H H. and Fortin M-J. (2005) Spatial analysis of landscapes: concepts and statistics, Ecology, Vol. 86(8), 1975-1987.
Warner T A. and Steinmaus K. (2005) Spatial classification of orchards and vineyards with high spatial resolution panchromatic imagery, Photogrammetric Engineering and Remote Sensing, Vol. 71(2), 179-187.
Warren M S. and Key R S. (1991) Woodlands: Past, Present and Potential for Insects, in Collins N M. and Thomas J A. (eds.), The Conservation of Insects and their Habitats, Harcourt Brace Jovanovich, London, 155-203.
Webb A., Buddle C M., Drapeau P. and Saint-Germain M. (2008) Use of remnant boreal forest habitats by saproxylic beetle assemblages in even-aged managed landscapes, Biological Conservation, Vol. 141(3), 815-826.
Weedon J T., Cornwell W K., Cornelissen J H C., Zanne A E., Wirth C. and Coomes D A. (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species?, Ecology Letters, Vol. 12, 45-56.
Wermelinger B., Flückiger P F., Obrist M K. and Duelli P. (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges, Journal of Applied Entomology, Vol. 131(2), 104-114.
Whitehead P F. (1992) Fruit trees, orchards and their rare invertebrates, Entomologist’s Gazette, Vol. 43, 303-304.
Whitehead P F. (1997) Invertebrates of pome and stone fruit orchards in the English Wyre Forest, July 1997, with particular regard to Gnorimus nobilis (L.,1758) (Col., Scarabaeidae), 1-8, Unpublished report for English Nature.
Whitehead P F. (2003) The noble chafer Aleurostictus nobilis (L., 1758)(Col., Scarabaeidae) in Britain, Proceedings of the second pan – European conference on saproxylic beetles, PTES, London.
Whiting (2002) Phylogeny of the holometabolous insect orders: molecular evidence, Zoologica Scripta, Vol. 31(1), 3-15.
Widdicome R. (2008) Traditional orchards: threats and opportunities within the planning system for Herefordshire and Worcestershire, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 143-155.
Wilson E O. (2002) The Future of Life, Little Brown, London.
With K A. and Crist T O. (1995) Critical threholds in species’ responses to landscape structure, Ecology, Vol. 76(8), 2446-2459.
With K A., Cadaret S J. and Davis C. (1999) Movement responses to patch structure in experimental fractal landscapes, Ecology, Vol. 80, 1340–1353.
Woodman J D., Cooper P D. and Haritos V S. (2007) Effects of temperature and oxygen availability on water loss and carbon dioxide release in two sympatric saproxylic invertebrates, Comparative Biochemistry Part A: Molecular and Integrative Physiology, Vol. 147(2), 514-520.
Worcestershire Biodiversity Partnership (2008) Worcestershire Biodiversity Action Plan 2008: S5 Noble Chafer SAP, Worcestershire Biodiversity Partnership Publications, Worcester.
Wrigley E A. (1985) Urban growth and agricultural change: England and the continent in the early modern, The Journal of Interdisciplinary History, Vol. 15(4), 683-728.
Wright D H. (1983) Species-energy theory – an extension of the species area theory, Oikos, Vol. 41, 496-506.
Wu J., Yu X-D., Zhou H-Z. (2009) The saproxylic beetle assemblage associated with different host trees in Southwest China, Insect Science, Vol. 15(3), 251-261.
Wünsch A. and Hormaza J I. (2002) Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers, Euphytica, Vol. 125(1), 59-67.
Yee M., Growe S J., Richardson A. and Mohammed C L. (2006) Brown rot in inner heartwood: why large logs support characteristic saproxylic beetle assemblages of conservation concern, in Grove S J. and Hanula J L. (eds.), Insect biodiversity and dead wood: proceedings of a symposium for the 22nd International Congress of Entomology, U.S. Department of Agriculture, Forest Service, Southern Research Station, Asheville, 42-56.
Zdenĕk B P., Turčáni M. and Horák J. (2012) Sharing the same space: foraging behaviour of saproxylic beetles in relation to dietary components of morphologically similar larvae, Ecological Entomology, Vol. 37, 117-123.
Zuur A F., Ieno E N. And Elphick C S. (2010) A protocol for data exploration to avoid common statistical problems, Methods in Ecology and Evolution, Vol. 1(1), 3-14.
Images
01. Factors influencing the presence of Gnorimus nobilis.