The autecology of the Noble Chafer Gnorimus nobilis within Worcestershire
Jenni Schenke
[A summary of a project undertaken for MSc in Countryside Management, Manchester Metropolitan University]
1. Introduction
The Noble Chafer (Fig. 1.) is a relatively large and distinctive metallic green beetle which I am sure many Worcestershire Record readers will be aware of thanks to the numerous appeals for sightings made to readers over the years from Harry Green (‘An edition of Worcestershire Record without Noble Chafer is almost unthinkable!’ (April 2007). However, just in case you would like a quick reminder or you are a new reader I thought I should include some brief background information on the species.
Noble Chafers are saproxylic (dependent upon dead/decaying timber) as larvae, feeding upon the wood mould of trees (Alexander, 2008a; Mann, 2006). Fortunately this makes searching for the species relatively easy as larvae produce distinctive faecal pellets known as frass which can be surveyed for all year around. Usually the elusive beetles develop after two years and if you are lucky you can find them nectaring upon pale/white flowers such as hogweed and meadowsweet on hot summer days (although interestingly this year Simon Wood discovered one on a plum trunk on a rather grey June day).
The species is considered to be an old forest relic (Alexander, 2002b; Whitehead, 2000) having moved over time from pioneer birch and willow, to oak trees, to cultivated fruit trees within traditional orchards which it has been proposed may be a response to climate change (Whitehead, 2003). Unfortunately the shift towards cultivated fruit trees may have been a bad move for the Noble Chafer as their saproxylic nature means that they require the dead/decaying wood associated with mature, well-spaced, open-grown trees. Changes in farming practices have meant that cultivating such trees is no longer economically viable and many traditional orchards have been grubbed up, replaced with dwarf/bush trees or destroyed by development. The situation is such that traditional orchards are now one of the rarest Biodiversity Action Plan (BAP) priority habitats). I t is therefore not surprising to find that the English range of Noble Chafers has declined significantly and that considerable concern exists for the survival of the species.
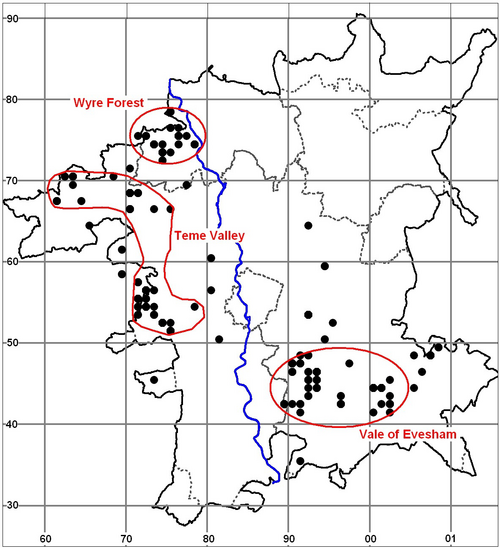
The main population stronghold has become largely confined to the Severn Basin (Herefordshire, Worcestershire and Gloucestershire) (Figs. 2. & 3.) with Worcestershire appearing to be a particular hotspot. Outlying populations exist in south Oxfordshire, Kent and interestingly the New Forest where the beetle has been found outside of traditional orchards (maps available on NBN Gateway). The Vale of Evesham and Wyre Forest are thought to contain two of the population centres within Worcestershire, with the Teme Valley area also appearing to be a favoured area (this has become more apparent recently since the Teme Valley Orchards Project) (Fig. 4.). There is potential for the Teme Valley and Wyre Forest areas to form one large centre, but further survey work is required to determine this.
Conservation of the Noble Chafer is important both locally and nationally (Worcestershire/UK Biodiversity Action Plan (BAP) species, Red Data Book 2), and also within most of the rest of its European range where it is largely considered to be of conservation concern. With its distinctive appearance, ease of survey and conservation importance it is not surprising that the Noble Chafer has become somewhat of an icon for conservation of the traditional orchards it inhabits and other species associated with such habitats.
My study aimed to build upon the annual PTES survey work, which have reinforced concern for the species’ survival (Green, 2004), and find out more about the habitat preferences of the Noble Chafer in the hope that findings could be used to direct future research and conservation. In order to achieve this, comments made by May (1994) that ecological studies must be pursued at many different levels i.e. spatial scales in order to understand what is going on ecologically, were taken on board and formed the basis of the study. Therefore environmental variables were investigated at host-plant, site, and landscape scales in an effort to discover factors and scales influential on Noble Chafer distribution.
2. Methods
All known existing records of Noble Chafer within Worcestershire were mapped and overlaid across the national traditional orchards habitat layer created by PTES so that potential survey sites could be short-listed.
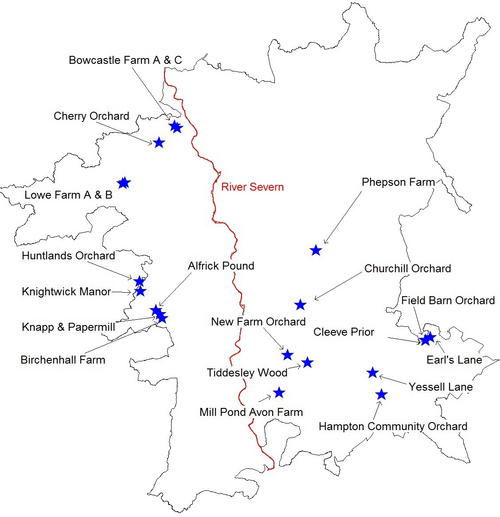
The River Severn naturally dissects Worcestershire and was used to form two study areas; east and west. Ten short-listed sites were selected from each area for survey, including a variety of different orchards i.e. those with sparse and multiple records, isolated and well-connected orchards, private and protected sites (Fig. 5., Table 1).
Tree and orchard characteristics data were collected in the field while landscape scale data was obtained using Mapinfo GIS. Evidence of Noble Chafer was determined using the accepted method of searching for larval frass using a long handled spoon. This method is considered much less damaging to the larval pabulum than searching for larvae (Smith, 2003; Whitehead, 2003) and is much easier to identify than the larvae themselves (Alexander 2004b; Hammond & Hine 1999). Also frass can be found throughout the year unlike the elusive adult beetles which are rarely observed (Alexander 2002b; Mann 2006; Whitehead 2003).
The data was analysed using various statistical tests including Independent samples t-tests, Chi-square, Regression tree analysis, Oneway Analysis of Variance and the G-test of independence.
| Name | Grid Ref | Size(ha) | Description |
East Area | |||
| Churchill Orchard | SO922532 | 0.46 | Mature plum orchard; many trees dead and fallen |
| Cleeve Prior Community Orchard | SP076488 | 1.29 | Managed by Cleeve Prior Heritage Trust; apple & plum |
| Earl’s Lane Orchard | SP082492 | 1.47 | Managed by Vale Landscape Heritage Trust; apple & plum |
| Field Barn Orchard | SP075488 | 0.32 | Plum orchard adjacent to Cleeve Prior Community Orchard |
| Hampton Community Orchard | SP020421 | 0.85 | Managed by Vale Landscape Heritage Trust; plum |
| Mill Pond Avon Farm | SO895423 | 0.51 | Derelict and overgrown apple orchard |
| New Farm Orchard | SO905470 | 1.38 | Mixed orchard |
| Phepson Farm | SO942599 | 0.14 | Derelict damson orchard, isolated noble chafer site |
| Tiddesley Wood | SO930461 | 0.62 | Wildlife Trust reserve; plum and damson near to ancient woodland |
| Yessell Lane Orchard | SP010448 | 7.55 | Soon to be in Higher Level Stewardship; large plum |
West Area | |||
| Alfrick Pound; orchard 2 | SO743525 | 0.78 | Plum orchard with very high density of noble chafer |
| Birchenhall Farm | SO750515 | 2.89 | Large cherry orchard close to woodland reserves |
| Bowcastle Farm; A | SO769750 | 1.65 | Cherry orchard on bank within SSSI |
| Bowcastle Farm; C | SO766753 | 1.96 | Mixed orchard within SSSI adjacent to woodland |
| Cherry Orchard, Blissgate | SO746732 | 0.86 | Cherry orchard on steep bank next to SSSI |
| Huntlands Farm | SO723561 | 2.42 | Apple & plum orchard next to Leigh Brook |
| Knapp & Papermill | SO748520 | 1.65 | Worcestershire Wildlife Trust reserve; apple |
| Knightwick Manor; 2 | SO723549 | 1.67 | Cherry & plum orchard within cluster of old orchards |
| Lowe Farm; A | SO702682 | 2.02 | Apple orchard (close to orchard B) |
| Lowe Farm; B | SO704683 | 1.47 | Plum orchard on steep slope |
Table 1. Summary of orchard sites surveyed
3. Results - summary of significant findings (Table 2)
| Variable | Significant | Comments |
Host Plant Scale | ||
| Girth (at breast height) | Yes | Apple & plum trees with NC evidence had significantly greater girths than those without. Cherry trees exhibited the opposite |
| Species | Yes | The effect of tree species appeared to be reflected across several host plant variables |
| Proximity to next tree | Yes | Damson trees with NC evidence were closer to nearest fruit tree than those without |
| Proximity to next NC tree | No | Possible indicator of dispersal ability. NC can fly >700m to nectar but can also spend its life within a tree without ever leaving it |
| Tree density within 10m | Yes | NC presence was positively associated with tree density |
| Tree health (alive/dead/hollowing etc.) | Yes | Cherry trees with NC evidence had higher % dead branches |
| Rot hole (size/orientation etc.) | No | Weak associations for higher holes & South/West orientations. Recording temp. within rot holes may have been more useful |
| Height | Yes | Cherry trees with NC evidence were shorter than those without |
| Location within orchard | No | Thought to influence micro-climate & sun-exposure. Weak +ve association with bottom of slopes (possibly more sheltered) |
Site Scale | ||
| Area | Yes | Smaller orchards had a positive association with high NC tree density |
| Tree density | Yes | Orchards with highest fruit tree density had highest densities of NC trees |
| Dominant tree type | Yes | Stone fruits were positively associated with high NC tree density |
| Average tree girth | No | Not a good indicator of tree age/dead wood availability as high density NC orchards were mostly composed of plums/damsons which are much smaller than apples & cherries. More indicative of fruit species composition |
| Aspect, Elevation & Topography | No | Thought to influence shelter/sun-exposure but anthropogenic nature of orchards may override the effects of such variables e.g. orchards were probably originally planted in warmer more sheltered parts of people’s land and therefore such variables would be very similar across visited sites. May have been more significant difference if sites without NC were also included in study. |
| Orchard management | No | Boundary, vegetation, grassland & tree management thought to influence various environmental factors mostly related to micro-climate, availability of food supplies & nectar sources. Possible indication that these factors may not be as influential at site-scale. |
| Adjacent habitat | Yes/No | Sites located next to known NC orchards had significantly higher NC tree densities. Other habitat types had less of an effect |
Landscape Scale | ||
| Proximity to ancient woodland | No | Thought to be important due to NC’s historical association with woodland. However recording past and present woodland areas may have been more relevant |
| Location in relation to River Severn | No | Predicted would have impact on habitat preferences of NC due to different geographical factors e.g. climate, local distinctiveness. Also proposed as a dispersal barrier by Alexander, 2005, this may not be the case in Worcestershire. |
| Surrounding traditional orchard density | No | Recorded as an indicator of habitat connectivity, but data probably not representative as only orchards were included whereas other habitats may provide suitable connecting habitats i.e. rides in woodland may provide nectaring sites |
| Surrounding NC orchard density | Yes | Low density NC orchards had larger areas of NC orchards within 2.5km radius than those with intermediate NC tree density |
Table 1 – Summary table of variables investigated, their significance and brief explanation of results.
4. Discussion of significant findings
4.1 Host-plant scale
Trunk girth
Trunk girth was a significant characteristic in all but the damson trees recorded (damson were the least frequently recorded fruit species and had more been sampled the result may have become significant). These findings are comparable with other studies where increased trunk girth was to be positively related to saproxylic invertebrate presence and diversity (Alexander 2002; Grove,2002; Lindhe & Lindelöw 2004; Lush et. al. 2009; Oleksa 2009; Ranius 2002; Ranius & Jansson 2000).
Trunk girth is a key factor influencing Noble Chafers as girth increases as the tree ages, therefore there is likely to be more deadwood, fungal decay and rot holes in larger girthed trees (Alexander 2008b; Green 2004; Ranius 2002) (providing they are not hollow). Larger girthed trunks will also have a relatively smaller surface area compared to smaller counterparts and so in theory will be better at retaining heat and providing a more stable environment retaining heat and better moisture conditions suitable for larval development (Alexander, 2008b; Begon et. al., 1990; Schiegg, 2001; Ranius & Jansson, 2000; Schiegg, 2001) for the ‘warmth-loving’ Noble Chafer (Alexander, 2008b).
The cherry tree result is interesting and requires further investigation as Noble Chafer trees had significantly smaller girths. Out of all the trees surveyed, the cherries were by far the tallest and largest and displayed the ‘healthiest’ appearance (solid trunks, fewer rot-holes). It may be that cherry trees rot differently and rot holes may occur higher up in the branches which were not searched. Bark thickness can also be a limiting factor for saproxylic species (Abrahamsson & Lindbladh, 2006; Buse et. al., 2007; Hedgren, 2007) and with bark thickness and trunk diameter being highly correlated it is possible that smaller cherry trees may be more penetrable.
Tree species
Noble Chafer evidence was only recorded in four fruit species all belonging to either Prunus or Malus genus thus agreeing with Whitehead’s comment in 2003 that ‘[Noble Chafer] seem to prefer senile Prunus & Malus species’. The narrow range of host species recorded and relatively strong preference for plum trees indicates a rather specialised nature, typical of saproxylic species (Alexander 2008a, Alexander 2004a; Bouget et. al. 2008b; Dodelin 2008). However, this survey was restricted to orchards and no other fruit species were recorded (it is possible that Worcs contains a relatively high proportion of plum trees).
Preferences for certain host species may be explained by differences in microhabitat dynamics (Dubois et. al. 2009b; Ranius 2002) i.e. the amount of wood mould, aspect of rot-holes (Ranius 2002; Ranius & Nilsson 1997) which may be the result of natural characteristics i.e. growth speed, wood quality; or management e.g. pruning (Dubois et. al. 2009b, Lachat et. al. 2006). Therefore investigating tree characteristics e.g. bark depth, rot-hole temperature/moisture conditions within different regions of the species’ UK and European range may provide more answers explaining host tree preferences and the geographic differences that occur (i.e. traditional orchards within Britain (with the exception of the New Forest) and a wider range of broadleaved species on the continent).
Proximity to other fruit trees
This was recorded as an indicator of micro-climate with the assumption that the more widely spaced trees would receive the greatest amount of sun-exposure (Dubois et. al.,2009b; Green 2004) and would be more likely to provide a warmer larval pabulum, aiding survival and development of larvae (Alexander 2002b; Green 2004; Whitehead, 2003). Sun-exposure of the host wood substrate is known to positively influence saproxylic invertebrate presence and can be of more importance than trunk diameter (Buse et. al. 2007; Djupström et. al.,2008; Dubois et. al. 2009b; Lindhe & Lindelöw 2004; Lindhe et. al. 2005; Ranius & Nilsson 1997).
Interestingly the opposite association to that expected was apparent where trees without Noble Chafer evidence had the most open space around them. Occupied trees were also closer to the nearest tree than unoccupied trees (except apple which showed a very weak opposite trend). These results suggest that Noble Chafer preferred semi-exposed conditions similar to findings by Jonsell et. al. 2004, Ranius 2000 and Ranius 2002. Such conditions may be more suitable for fungal activity and rot-hole development (Bouget & Duelli 2004; Lindhe et. al. 2004). The smaller size of fruit trees (as opposed to larger broadleaves often included in saproxylic studies) may mean that some shading helps to stabilise internal trunk conditions. Another factor to consider is that orchards are planted to maximise fruit productivity with full canopy growth therefore trunks may still receive relatively high amounts of sun-exposure even if other trees are nearby. They are also often planted in sheltered, sunny or south-west facing areas (Wedge 2007b) and sometimes pruned (Green 2004). Alternative methodology i.e. insolation index based on sunshine intensity, measuring evaporation (Buse et. al. 2007; Lindhe & Lindelöw 2004) may have provided better comparison of micro-climate.
Proximity to other Noble Chafer trees
Distance to nearest Noble Chafer tree was not significantly different between occupied and unoccupied trees for any tree species. This may suggest that Noble Chafers are good dispersers and is comparable to the findings by Gibb et. al. 2006a who found that specific red-listed forest beetles had lower wing-loadings and superior flying ability than more common species. The fact that Noble Chafers have been observed nectaring and copulating on flowers up to 700metres away from known orchard sites (Alexander 2004b; Whitehead 2003) indicates that they have the ability to disperse relatively far away to find suitable host trees.
However, Noble Chafers are generally only observed flying when temperatures are really warm (Alexander 2002b; Mann 2006; Whitehead 2003) and it is proposed that they can spend their whole life within one tree without leaving it at all (Whitehead 2003). This also appears to fit with the findings that distance to nearest host tree is not significant.
It is therefore difficult to establish the scale at which metapopulations exist i.e. site scale or landscape scale (Krauss et. al., 2005). Ranius, 2006 appeared to have the same difficulty in his study of O. eremita. He proposed that the extremely low dispersal rate between trees could mean that each tree hosted a local population, making the stand of trees a metapopulation. He then later suggested that metapopulation dynamics may also arise at a larger scale, with each stand of hollow trees sustaining a local population and the individuals in an entire landscape forming a metapopulation. Perhaps, as proposed by Krauss et. al. 2004 in their study of the Small Blue Butterfly Cupido minimus Fuessly, the majority of Noble Chafers are sedentary and only a few adults disperse widely, helping to maintain genetic diversity within local populations (Harrison 1993; Ranius 2006). Dispersal is a key characteristic in species conservation and major contributor to metapopulation viability (Dubois et. al. 2009a) therefore further investigation into the dispersal ability of this elusive beetle is required.
In contrast to this study’s findings Buse et. al. 2007,Grove, 2002 and Ranius 2006 all found distance to nearest occupied tree to be a significant host plant-scale factor. However Buse et. al. 2007 classified distance to next nearest host plant as a landscape-scale factor as opposed to host plant-scale which demonstrates the difference between orchard habitats which in theory have higher microhabitat densities (i.e. more potential host trees with more rot-holes) than isolated oak trees (Dubois et. al. 2009b).
Tree condition
Findings indicate that Noble Chafer trees had higher percentages of dead branches than those without Noble Chafer evidence, although this difference was only significant between cherry trees (and very nearly apple trees). These findings are comparable with studies by Buse et. al. 2007 and Lachat et. al.,2006 where the proportion of dead branches was found to be a positive indicator of saproxylic invertebrates. Nordèn et. al. 2004 also recognised the importance of attached dead wood but recommended that further investigation of its biodiversity value was required. Proposed reasons for this positive association include greater sun-exposure to the trunk with higher numbers of dead branches giving less shading from foliage (Green, 2004), and more opportunities (i.e. rot-holes) to enter the tree (Alexander, 2004b).
Tree height
The effect of host-plant height on invertebrates has been well documented; however there has been less study of the effect of tree height on saproxylic species. In this study only cherry trees showed a significant difference in height and similarly to other factors investigated (girth, rot-hole height and distance to nearest occupied tree) exhibited the opposite trend to the other fruit species i.e. shorter trees were preferred. This may be because cherries were the largest of the fruit trees investigated and so it is possible that shorter trees are preferred as they represent the weaker, more accessible specimens (i.e. reduced bark thickness).
4.2 Site Scale
Fruit tree density and orchard area
Orchards with the highest fruit tree densities had significantly higher densities of Noble Chafer trees, a finding which is frequently found in invertebrate studies (Krauss et. al. 2005; Krauss et. al. 2004; Müller et. al. in press) because a greater food resource will support more individuals (Yee et. al., 2007).
These findings may again reflect the importance of tree species at site scale (Dubois et. al. 2009b; Oleksa et. al. 2007) as orchards dominated by plum had the highest fruit tree densities. This may also indicate a preference for warmer, more stable microclimates as higher densities of fruit trees may provide shelter against wind and retain heat thus speeding up larval development (Green 2004).
Habitat patch size is frequently strongly and positively correlated with host-plant density (Krauss et. al. 2005) and invertebrate density with larger patches providing more resources and having lower extinction risks (Krauss et. al. 2004; Ranius 2002; Sahlin & Schroeder 2010; Tikkanen et. al. 2009). However, in comparison this study found that smaller orchards had the highest Noble Chafer densities. This finding may again be linked to fruit tree composition as plum trees, (the preferred species) are much smaller than apple and cherry trees meaning a greater number can be planted in a smaller area.
Whitehead (2003) noted the resilience of the Noble Chafer and its ability to form small-scale populations in restricted areas of acceptable habitat. Therefore it is possible Noble Chafers can live in high densities where habitat quality is optimal even if the sites are relatively small. However if this is the case then these sites will need to have good links with other favourable habitats (Kindlmann et. al. 2005; Krauss et. al. 2005; Webb & Thomas 1994), a number of dispersive individuals (Dubois et. al. 2009a; Kindlmann et. al. 2005; Krauss et. al. 2004) or a continued supply of mature fruit trees (Alexander,2008b; Lush et. al. 2009; Webb et. al. 2010) otherwise the populations will eventually collapse.
Adjacent habitats
Adjacent habitats were recorded because they are believed to have an important microclimatic role on the sites invertebrates inhabit (Dubois et. al. 2009b; Dubois & Vignon 2008; Lindbladh et. al. 2003; Lindhe et. al 2005; Ranius & Nilsson,1997). The strong positive association between adjacent orchards and the relatively strong negative association with woodlands could be seen to support the microclimate theory even though they exhibit opposite trends. This is because although both would provide shelter, the more widely spaced trees in orchards would also allow the sun to penetrate thus creating more suitable conditions (Dubois et. al. 2009b; Green 2004) i.e. warmer rot holes to aid larval maturation (Alexander 2002b; Green 2004; Whitehead 2003), warmer air temperatures to aid dispersal (Dubois et. al. 2009a).
The positive association with adjacent orchard habitats could also be linked to the increased the patch size and reduced edge effects (Johansson et. al. 2007; Johnson et. al. 2002).
It is also possible the negative woodland association supports the theory that adjacent habitats affects dispersal efficiency as the tall, dense surrounding vegetation creates an obstacle for movement (Dubois et. al, 2009b; Dubois & Vignon 2008; Jeanneret et. al, 2003; Kindlmann et. al. 2005).
4.3 Landscape Scale
Density of Noble Chafer orchards in surrounding area
It is well known theory that the distribution and continuity of suitable habitats is a limiting factor for invertebrate populations and that the most well-connected sites support the greatest populations due to factors such as high rates of immigration and low extinction risk (Krauss et. al. 2005; Lush et. al. 2009; Ranius 2002; Sahlin & Schroeder 2010; Sverdrup-Thygeson et. al. 2010; Webb,2006; Webb & Thomas 1994).
Low density orchards had the greatest areas of Noble Chafer orchards at both scales; the effect being more pronounced at the larger scale (2.5km) (analysis of surrounding traditional orchards produced similar patterns, but were not significant). This result is interesting and may indicate the negative effect of habitat fragmentation whereby more isolated sites become more densely populated because only a small proportion of the population is able to disperse to more distant orchards (although this proportion may be greater than that of low density orchards) thus supporting some of the metapopulation theories mentioned earlier and highlighting the importance of the species’ dispersal ability.
The importance of dispersal ability for determining the scale at which metapopulation dynamics such as connectivity becomes relevant has been raised by a number of studies (Krauss et. al. 2004; Krauss et. al. 2005; Ranius, 2002). Findings by other studies suggest that the Noble Chafer’s dispersal ability could be relatively good due to the beetle’s relatively large size (Webb et. al. 2006) and distribution within fragmented habitats (Merckx & Dyck 2007). Therefore further investigation into the Noble Chafer’s dispersal ability may provide useful information.
However, it is also important to consider that occurrence patterns may also be related to historical habitat distribution i.e. before agricultural intensification when orchards were less fragmented and isolated (Lush et. al. 2010; Ranius 2002; Sahlin & Schroeder 2010). Therefore the landscape matrix may have become more inhospitable during this time with fewer corridors and stepping stones (Krauss et. al.,2005; Tikkanen et. al. 2009; Webb 2006) as Alexander (2004a; 2005b) believed to be the case in Gloucestershire. Further analysis including the comparison of historical OS maps with present orchard distribution may provide information to support/reject this theory.
Also the species distribution data used in this study is limited to the holdings of Noble Chafer records by Worcestershire Biological Records Centre at the time of the study. Therefore some positive orchards may have been missed because they had not been surveyed or because records had not been supplied to WBRC. Although it is difficult to conclusively determine negative Noble Chafer orchards/trees, more robust testing of this theory would require the survey of every orchard in both radii around sites to determine both presence/absence as well as Noble Chafer density. Potential nectaring sites should also be included as they can be important breeding sites (Alexander 2004b; Whitehead 2003) and may also provide important stepping stones between apparently isolated orchards.
5. Summary
Factors affecting Noble Chafer distribution occurred across all spatial scales with host-plant appearing to be the most influential (trunk girth being particularly important). I t is proposed that this may reinforce the highly specialised nature of saproxylic species and importance of the dead wood provided by host-plants, although biases within the study meant this cannot be decisively concluded. The findings do demonstrate the complex nature of interactions that exist between Noble Chafers and traditional orchards, and the interconnectedness of factors between scales. Finding out more about the dispersal ability of Noble Chafer and investigating a greater range of landscape-scale variables is essential for informing long-term conservation management. In the mean time ensuring that the landscape provides a mosaic of well-connected traditional orchards and nectaring sites is important.
6. Recommendations
Further research
Developing a more reliable non-invasive method to determine presence/absence of Noble Chafer would be extremely useful. Deborah Harvey’s current study (part funded by PTES) on nitrogen fixation of the Stag Beetle, Noble Chafer and Variable Chafer may provide some leads for this kind of development in the future.
Recording tree characteristics in more detail i.e. rot-hole temperature, moisture could help to explain host-plant preferences.
Surveys which determine both presence/absence and assess habitat suitability for Noble Chafer (using Alexander s 2002a grading system) should continue and the data should be mapped using GIS to enable large scale analysis.
Mapping other important habitats e.g. nectaring sites, historical orchards may also produce some interesting results and help to inform long-term conservation management i.e. identifying linkages/obstacles between sites (Alexander, 2002a), and priorities for conservation i.e. isolated orchards.
Finding out more about the dispersal ability of Noble Chafer will also be essential, but could prove rather challenging due to the elusive nature of the beetle!
Noble Chafer conservation
Apply a landscape-scale approach maintaining a mosaic if well connected habitats (Alexander,2002a; Webb et. al.,2010; Webb 2006; Wedge 2007c) e.g. creating rides through woodlands separating orchards.
Increase the availability of mature fruit trees in the surrounding landscape by establishing replanting programmes, especially within known Noble Chafer orchards (Lush et. al. 2009).
Provide continued encouragement for landowners to participate in agri-environment schemes so that existing orchard habitats are protected and enhanced (Alexander, 2002a). Hopefully the inclusion of traditional orchards as a priority in the BAP Habitat Action Plan will help to ensure that this happens (Lush et. al. 2009).
Hopefully conservation action applied across a range of scales as suggested should not only help to conserve the Noble Chafer, but also numerous other important wildlife species which have come to depend on such habitats.
Acknowledgements
Thank you to everyone who helped me during my project.
References
Abrahamsson, M. and Lindbladh, M. 2006. A comparison of saproxylic beetle occurrence between man-made high- and low-stumps of spruce (Picea abies). Forest Ecology and Management. 226 (1-3):230-237.
Alexander, K. N. A. 2002a. The Noble Chafer Gnorimus nobilis in Worcestershire – a report on the 2002 survey. Unpublished report for The People’s Trust for Endangered Species.
Alexander, K. N. A. 2002b. The invertebrates of living and decaying timber in Britain and Ireland – a provisional annotated checklist. Research Report No. 467, English Nature, Peterborough,.
Alexander, K. N. A. 2004a. The Noble Chafer Gnorimus nobilis in Gloucestershire – a report on the 2004 survey. Unpublished report for People’s Trust for Endangered Species.
Alexander, K.N.A. 2004b. Revision of the Index of Ecological Continuity as used for saproxylic beetles. Research Report No. 574. English Nature Peterborough.
Alexander, K. N. A. 2008. Tree biology and saproxylic Coleoptera: Issues of definitions and conservation language. In: Vignon V.,and Asmodé J.-F. (Eds). Proceedings of the 4th Symposium on the Conservation and Workshop of Saproxylic Beetles 27-29th June 2006. p. 1-6. Vivoin Sarthe Department, France.
Begon, M., Harper., J.L. and Townsend, C.R. 1990. Ecology Individuals, Populations and Communities (2nd ed.). Blackwell Scientific Publications, Oxford
Bouget, C., Brustel, H. and Zagatti, P. 2008b. The French Information System on Saproxylic Beetle Ecology (FRISBEE): an ecological and taxonomical database to help with the assessment of forest conservation status. In: Vignon V., Asmodé J.-F. (Eds). Proceedings of the 4th Symposium on the Conservation and Workshop of Saproxylic Beetles 27-29th June 2006. p. 25-28. Vivoin Sarthe Department, France.
Bouget, C. and Duelli, P. 2004. The effects of windthrow on forest insect communities: a literature review. Biological Conservation 118:281–299.
Buse, J., Schröder, B., and Assmann, T. 2007 Modelling habitat and spatial distribution of an endangered longhorn beetle – A case study for saproxylic insect conservation. Biological Conservation. 137 (3):372-381.
Djupström, L.B., Weslein, J. and Schroeder, L.M. (2008) Dead wood and saproxylic beetles in set-aside and non set-aside forests in a boreal region. Forest Ecology and Management 255 (8-9):3340-3350
Dodelin, B. 2008 Aspects of the repartition of the saproxylic beetles in forests (French Alps). In: Vignon V., Asmodé J.-F. (Eds). Proceedings of the 4th Symposium on the Conservation and Workshop of Saproxylic Beetles. p. 47-52. Vivoin Sarthe Department, France
Dubois, G. and Vignon, V. 2008 First results of radio-tracking of Osmoderma eremita (Coleoptera: Cetoniidae) in French chestnut orchards. In: Vignon V., Asmodé J.-F. (Eds). Proceedings of the 4th Symposium on the Conservation and Workshop of Saproxylic Beetles 27-29th June 2006. p. 123-130. Vivoin Sarthe Department, France
Dubois, G.F., Vernon, P. and Brustel, H. 2009a. A flight mill for large beetles such as Osmoderma eremite (Coleoptera: Cetoniidae). In: Buse, J., Alexander, K.N.A., Ranius, T. and Assman, T. (Eds). Saproxylic beetles: their role and diversity in European woodland and tree habitats – proceedings of the 5th symposium and workshop 14-16th June 2008. p. 219-224. Leuphana University, Institute of Ecology and Environment, Luneburg, Germany.
Dubois, G.F., Vignon, V., Delettre, Y.R., Rantier, Y., Vernon, P. and Burel, F. 2009b. Factors affecting the occurrence of the endangered saproxylic beetle Osderma eremita (Scopoli, 1763) (Coleoptera: Cetoniidae) in an agricultural landscape. Landscape and Urban Planning 91 (3):152-159.
Gibb, H., Hjältén, J., Ball, J.P., Pettersson, R.B., Landin, J. and Alvini, O. 2006a. Wing loading and habitat selection in forest beetles: are red-listed species poorer dispersers or more habitat-specific than common congenerics? Biological Conservation 132:250–260
Green, G.H. 2004. The noble chafer Gnorimus nobilis – Records needed! Worcestershire Record 16:10-11
Grove, S.J. 2002. Tree basal area and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics. Ecological Indicators. 1 (3):171-188.
Hammond, P.M. and Hine, S.J. 1999. Coleoptera; the beetles. In: Barnard, P.C. (Eds.) Identifying British Insects and Arachnids An annotated bibliography of key works. Cambridge University Press, Cambridge. p. 80-138
Harrison, S. 1993. Metapopulations and conservation. In: Edwards, P.J., May, R.M. and Webb, N.R. (Eds.) Large-Scale Ecology and Conservation Biology. Blackwell Scientific Publications, Oxford p. 111-128
Hedgren, P.O. 2007. Early arriving saproxylic beetles (Coleoptera) and low and high stumps of Norway spruce. Forest Ecology and Management. 241 (1-3):155-161
Jeanneret, Ph., Schϋpbach, B. and Luka, H. 2003. Quantifying the impact of landscape and habitat features on biodiversity of cultivated landscapes. Agriculture Ecosystems and Environment 98:311-320
Johansson, T., Hjältén, J., Gibb, H., Hilszczanski, J., Stenlid, J., Ball, J.P., Alinvi, O. and Danell, K. 2007. Variable response of different functional groups of saproxylic beetles to substrate manipulation and forest management; Implications for conservation strategies. Forest Ecology and Management. 424 (2-3):496-510(2)
Johnson, C. M., Johnson, L.B., Richards, C. and Beasley, V. 2002. Predicting the Occurrence of Amphibians: An Assessment of Multiple-scale Models. In: Scott, M.J., Heglund, P.J., Morrison, M.L., Raphael, M.G., Wall, W.A. and Samson, F.B. (eds.). Predicting species occurrences: issues of accuracy and scale. Island Press. Washington. p.157-170
Jonsell, M., Nittèrus, K. and Stighäll, K. 2004. Saproxylic beetles in natural and man-made deciduous high stumps retained for conservation. Biological Conservation 118 (2):163-173
Kindlmann, P., Aviron, S. and Burel, F. 2005. When is landscape matrix important for determining animal fluxes between resource patches? Ecological Complexity 2 (2):150-158
Krauss, J., Steffan-Dewenter, I., Mϋller, C.B. and Tscharntke. 2005. Relative importance of resource quantity, isolation and habitat quality for landscape distribution of a monophagous butterfly. Ecography. 28 (4):465-474
Krauss, J., Steffan-Dewenter, I. and Tschanrntke, T. 2004. Landscape occupancy and local population size depends on host plant distributions in the butterfly Cupido minimus. Biological Conservation 120 (3):355, 361.
Lachat, T., Nagel, P. Cakpo, Y., Attignon, S., Goergen, G., Sinsin, B. and Peveling, R. 2006. Dead wood and saproxylic beetle assemblages in a semi-deciduous forest in Southern Benin. Forest Ecology and Management 225 (1-3):27-38.
Lindhe, A. and Lindelöw, Å. 2004. Cut high stumps of spruce, birch, aspen and oak as breeding substrates for saproxylic beetles. Forest Ecology and Management. 203:1-20.
Lindhe, A., Åsenblad, N. and Toresson, H-G. 2004. Cut logs and high stumps fo spruce, birch, aspen and oak – nine years of saproxylic fungi succession. Biological Conservation 119 (4):443-454.
Lindhe, A., Lindelöw, Å. and Åsenblad, N. 2005. Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodiversity and Conservation. 14 (12):3033–3053.
Lush, M., Robertson, H.J., Alexander, K.N.A., Giavarini, V., Hewins, E., Mellings, J.,Stevenson, C.R., Storey, M. and Whitehead, P.F., 2009. Biodiversity studies of six traditional orchards in England. Natural England ,Peterborough. Natural Engand Research Report 025.
Mann, D.J. 2006. The Beetle Families Scarabaedeoidea. In: Cooter, J. and Barclay, M.V.L. (Eds.) A Coleopterist’s Handbook .4th ed. The Amateur Entomologists’ Society, Kent p.47-58.
Merckx, T. and Dyck, H. V. 2007. Habitat fragmentation affects habitat-finding ability of the speckled wood butterfly, Pararge aegeria L. Animal Behaviour 74 (4):1029-1037.
Müller, J., Noss, R.F., Bussler, H. and Brandl, R. (in press) Learning from a “benign neglect strategy” in a national park: Response of saproxylic beetles to dead wood accumulation. Biological Conservation.
Nordèn, B., Götmark, F., Tönnberg, M. and Ryberg, M. 2004. Dead wood in semi-natural temperate broadleaved woodland: contribution of coarse and fine dead wood , attached dead wood and stumps. Forest Ecology and Management 194 (1-3):235-248.
Oleksa, A. 2009. Conservation and ecology of the hermit beetle Osmoderma eremita s.l. in Poland. In: Buse, J., Alexander, K.N.A., Ranius, T. and Assman, T. (Eds). Saproxylic beetles: their role and diversity in European woodland and tree habitats – proceedings of the 5th symposium and workshop 14-16th June 2008. p. 177-188. Leuphana University, Institute of Ecology and Environment, Luneburg, Germany.
Oleksa, A., Ulrich, W. and Gawroński, R. 2007. Host tree preferences of hermit beetles Osmoderma eremita Scop., Coleoptera: Scarabaeidae in a network of rural avenues in Poland. Polish Journal of Ecology 55 (2):315-323.
Ranius, T. (2002) Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden. Biological Conservation 103 (1):85-91
Ranius, T. and Jansson, N. 2000. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biological Conservation 95:85-94.
Ranius, T. and Nilsson, S.G. (997. Habitat of Osmoderma eremita Scop (Coleoptera: Scarabaeidae), a beetle living in hollow trees. Journal of Insect Conservation 1, p. 193-204
Sahlin, E. and Schroeder, L.M. (2010) Importance of habitat patch size for occupancy and density of aspen-associated saproxylic beetles. Biodiversity and Conservation 19:1325-1339
Schiegg, K. 2001. Saproxylic insect diversity of beech: limbs are richer than trunks. Forest Ecology and Management 149 (1-3):295-304.
Smith, M. 2003. Noble Chafer Gnorimus nobilis Wyre Forest District, Worcestershire 2003 Survey Worcestershire. Unpublished report for People’s Trust for Endangered Species
Sverdrup-Thygeson, A., Skarpass, O. and Odegaard, F. 2010. Hollow oaks and beetle conservation: the significance of the surroundings. Biodiversity and Conservation 19 (3):837-852.
Tikkanen, O., Punttila, P. and Heikkila, R. 2009. Species-area relationships of red-listed species
Webb, J.R. 2006. Conservation. In: Cooter, J. and Barclay, M.V.L. (Eds.) A Coleopterist’s Handbook. 4th ed. The Amateur Entomologists’ Society, Kent p.47-58.
Webb, J.R., Drewitt, A.L., Measures, G.H. 2010. Managing for species: Integrating the needs of England’s priority species into habitat management. Part 1 Report. Peterborough: English Nature, NERR024.
Webb, N.R. and Thomas, J.A.1994. Conserving insect habitats in heathland biotopes: a question of scale. In: Edwards, P.J., May, R.M. and Webb, N.R. (Eds.) Large-Scale Ecology and Conservation Biology. Blackwell Scientific Publications, Oxford p. 129-151.
Wedge, C. 2007b. Traditional orchards: site and tree selection. Natural England, Bristol.
Wedge, C. 2007c. Traditional orchards: a summary. Natural England, Bristol.
Whitehead, P.F. 2003. The noble chafer Aleurostictus nobilis (L., 1758) (Col., Scarabaeidae) in Britain. In: People’s Trust for Endangered Species. Proceedings of the second pan-European conference on Saproxylic Beetles p.17-31. People’s Trust for Endangered Species, London.
Yee, D.A. and Juliano, S.A. 2007. Abundance matters: a field experiment testing the more individuals hypothesis for richness – productivity relationships. Oecologia 153 (1):153-162.
Images
Fig. 1. Fig. 1. The Noble Chafer Gnorimus nobilis. ©Harry Green
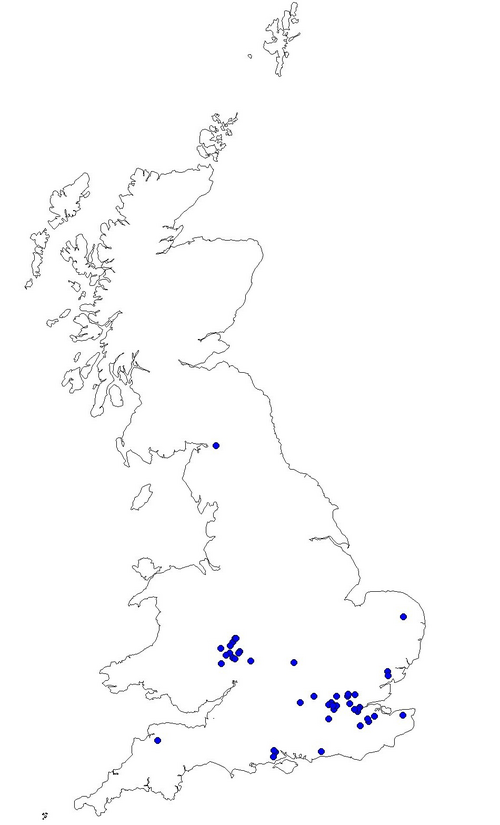
Fig. 2. Pre 1980 records of Noble Chafer Gnorimus nobilis in the UK. (data provided by PTES March 2010)
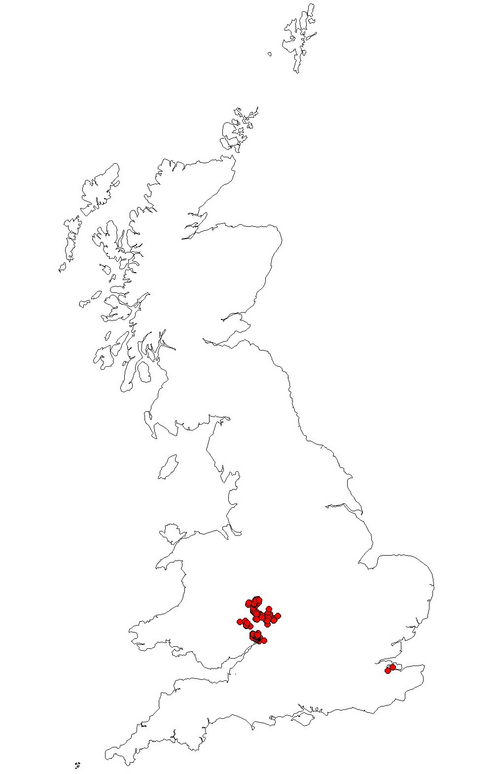
Fig. 3. Post 1980 records of Noble Chafer Gnorimus nobilis within the UK. (data provided by PTES March 2010)
Fig. 4. Noble Chafer Gnorimus nobilis distribution in Worcestershire - all records. (1901-2011) in relation to the River Severn (records provided by Worcestershire Biological Records Centre 2011)
Fig. 5. Orchards surveyed in Worcestershire for this project.