Investigating the habitat parameters of the Noble Chafer Beetle Gnorimus nobilis in the Wyre Forest area
Anna Bunney
Dissertation Summary
This dissertation was completed as part of the Geography and Environmental Management course at the University of the West of England. This is an abstract from the whole dissertation. Please contact Anna Bunney if you wish to read the full version.
The aim of this research was to investigate the orchard habitat preferences of the Noble Chafer Gnorimus nobilis beetle focussing on a comparison of orchard habitats where G. nobilis is present and orchards where it is absent. This was to be achieved by comparing traditional orchard habitats and their surrounding habitats, and determining which of them do and which do not support G. nobilis. The location of this study is the Wyre Forest area (Grid Reference: SO749765), which spans the Worcestershire and Shropshire border. The field research aimed to establish if there was a significant relationship between the presence of G. nobilis and individual habitat parameters. This dissertation specifically focuses on identifying differences in the tree species, tree girth, orchard microclimate, surrounding orchard habitat, and orchard management in relation to the presence or absence of G. nobilis in traditional orchards in the Wyre Forest area. Identifying these habitat parameters could help to sustain populations of G. nobilis in the future.
The research question was; “Are there any significant differences in orchard habitat and surrounding orchard habitat where the Gnorimus nobilis beetle is found to be present and where it is found to be absent?”
G. nobilis is one of the rarest saproxylic invertebrate beetles in Britain, labelled as “Vulnerable” by the UK BAP (United Kingdom Biodiversity Action Plan) (Smith 2005), with its remaining number unknown (Eccleson 2007). Due to its rarity, a BAP was implemented in 1999 (UK BAP, 2010).
It is recognised that the most influential factor for G. nobilis presence is dead wood, as it is a saproxylic invertebrate (Alexander 2008; European Red List 2010). Saproxylic invertebrates are dependent on dead wood (Speight 1989). G. nobilis larvae feed on accumulations of wood mould in tree cavities (Smith, 2005). Fruit trees in traditional orchards are relatively short lived, producing decaying wood more quickly than most native hardwood trees, making them important refuges for saproxylic invertebrates (Worcestershire Biodiversity Partnership, 2008).
Furthermore, it is also recognised that G. nobilis preferentially inhabits traditional orchards in the Severn Basin area of Worcestershire, Herefordshire and Gloucestershire (PTES 2010). A traditional orchard is defined as having at least five full sized fruit trees, planted on permanent grasslands, which are widely spaced and allowed to reach a veteran state (Berkshire Biodiversity Action Plan, 2010). However, since the 1950s, traditional orchards have been removed for urbanisation, agriculture, and other changes in land use (National Trust, 2010). It is thought that 85% of Worcestershire’s traditional orchards have been lost in the last 100 years (Worcestershire Biodiversity Partnership, 2008). Therefore the species associated with these habitats are being lost, posing a great threat to saproxylic species, including G. nobilis. Therefore, conservation of these habitats is crucial, otherwise species like G. nobilis will become extinct (UK Biodiversity Action Plan (BAP), 2010).
Sixteen orchards were sampled in total. Once the owner’s permission was granted, site visits were undertaken to determine the suitability of the orchards for this study. This was based on Alexander’s (2002) visual grading scheme for assessment of traditional orchards for G. nobilis. Only orchards that had the grade of one or two were taken on to research in the final study, as they were composed of the required tree species in the right condition to contain G. nobilis.
On this initial site visit, it was noticed that there was a significant difference in orchard size between study sites. Some orchards contained less than ten trees; however, most orchards were much larger, with over fifty trees. Sampling all the trees in all orchards could have taken a considerable amount of time, and it would not have been representative to compare these much larger orchards with the smaller orchards. Therefore, it was decided that for orchards with less than ten trees, all trees would be sampled. In orchards with more than ten trees, a random selection of ten trees was taken (because small orchards mostly had ten or fewer trees in them). It was considered to be more important to study fewer trees in detail, looking into all rot holes, split bark and crevices, than a large number of trees, when there would not be enough time to study the tree in detail. An accurate result of G. nobilis positive and negative trees, and therefore positive or negative orchards, would be obtained.
However, in some of these orchards, saplings were apparent, which had been planted by the land owner. These saplings were too young to contain any dead wood, so would not be a suitable habitat for G. nobilis (PTES, 2010). These younger trees were eliminated from the study, so that a random sample of trees old enough to contain dead wood, could be taken. Trees which had rot holes that were not accessible either because they were too deep or too high up to sample, even by ladder, were also eliminated from this study, as they could not be studied in enough detail.
Individual trees were given numbers, which were marked on the map. Site maps were created so that when the sampling was carried out, a random selection of trees in the orchards could be chosen. Random samples were carried out using Microsoft Excel. The individual tree numbers were entered in a row on a spreadsheet, and each tree was assigned a random number by the Microsoft Excel random number tool. This row was then sorted from smallest random number to largest random number, and the first ten trees with the lowest assigned random number were the trees that were selected for sampling.
As the PTES (2010) have identified, the best way of determining the presence of G. nobilis is by looking for its frass. This is an easy way of assessing presence, and causes no damage to the host tree, or any species inhabiting the tree. The pellets provide good evidence that the larvae have been living in the tree, and can be substantiated by seeing the larvae or parts of the dead beetle (PTES 2010). Larvae live deep within the tree hollows and are not always detectable, even when present. Searching for them could damage or destroy the host tree (Alexander, 2005), whereas frass can be studied without damaging the host tree (Smith 2005).
In this study, sampling was carried out by carefully scooping into any rot holes, crevasses, or behind any bark in the sample tree with an extended spoon to excavate a small amount of wood mould. In some cases, wood mould was easily accessible and G. nobilis frass was identifiable visually. However in most cases, the extended teaspoon was used to scoop into rot holes and crevasses in the tree to obtain a wood mould sample because the frass can be found quite deep within the tree. The wood mould sample was then spread out on a sheet of white paper to examine for frass. Frass is approximately 3mm long, and somewhat resembles mouse droppings (PTES, 2010). This process was repeated in every part of the sample tree where wood mould could have potentially been found.
When sampling for frass in the trees, it was easy to determine when there was a definite positive result if it resembled the frass seen in G. nobilis positive trees at Tiddesley Orchard (SO927462), Pershore, a G. nobilis positive orchard owned by the Worcestershire Wildlife Trust. The G. nobilis frass stood out well as it was much larger and the colour was either much darker or lighter (depending on age) than the wood mould or any other invertebrate frass, such as woodlouse frass. It was also easy to define a G. nobilis negative tree, as only very fine wood mould was found. However, in some cases, it was hard to be certain. When an unsure sample was taken from the tree, it was put in a sampling bag and sent to G. nobilis expert Harry Green for checking.
A second, but far less reliable way to determine G. nobilis presence was to look for adult beetles. However, as they are only in flight for four to six weeks in early summer, it is a rare occurrence to see them (Smith 2003). This research was undertaken in November so it was not a suitable or reliable method to assess G. nobilis presence.
All trees selected for sampling in the orchards were looked at thoroughly, in all hollows, rot holes and crevasses, to search for frass. Once all sample trees were examined, the orchards were classified as an orchard containing, or not containing G. nobilis. For example if one tree in an orchard of thirty trees contained G. nobilis frass, it would be classified as a G. nobilis positive orchard.
To investigate the habitat preferences of G. nobilis the following set of data was collected in all orchards:
Species of tree and tree girth at every randomly selected orchard tree.
Immediate adjacent habitat - agriculture (pesticides being applied or not), housing, orchards, woodland, industry, roads.
Management techniques applied within the orchard.
Orchard microclimate - Orchard aspect, tree density, orchard size.
Raw data for this report was collected in the field over a period of five days from the 1st November to the 5th November 2010. In total, 158 trees were sampled across sixteen orchard sites.
28 trees were observed as G. nobilis positive.
12 orchards were recorded as G. nobilis positive.
4 orchards were recorded as G. nobilis negative.
The highest number of G. nobilis positive trees was found at Cherry Orchard, Bowcastle Farm.
Previously unrecorded G. nobilis sites were found at Hole Farm, The Cottage, Dreamskerry, Far Forest School, Hawkbatch Farm, The Latchetts and Starrs Hill.
One orchard, Tarn, was recorded in previous Worcestershire Biological Records Centre surveys as G. nobilis positive, however in this study there was no evidence of G. nobilis in this orchard.
The results of this study are summarised in table 1.
| Habitat Parameter | Relationship with G. nobilis Presence? | Evidence |
| Tree Characteristics: | ||
| Tree Species - Tree Level | P. domestica preferred by G. nobilis | 33.33% of Prunus domestica trees contained G. nobilis. |
| Tree Species - Orchard Level | P. avium preferred by G. nobilis | 83.87% of Prunus avium trees were in G. nobilis positive orchards. |
| Statistical Analysis of Tree Species Preference | None | H0 was accepted - G. nobilis has no preference for particular orchard tree species. |
| Orchard Tree Species Composition | G. nobilis appear to prefer more diverse orchards | G. nobilis positive orchards contained a higher number of different tree species than in G. nobilis negative orchards. |
| Tree Girth - Tree Level | G. nobilis was found in trees with a larger mean tree girth, and a smaller tree girth range than G. nobilis negative trees. | Mean tree girth of G. nobilis positive trees was 138.29cm, whereas G. nobilis negative trees had a mean tree girth of 127.02cm. The range of tree girth was 155cm in G. nobilis positive trees, and 230cm in G. nobilis negative trees (see Discussion). |
| Tree Girth - Orchard Level | Not a considerable difference in mean tree girth between G. nobilis positive orchards and G. nobilis negative orchards. Tree girth range was lower in G. nobilis positive orchards. | Mean tree girth of G. nobilis positive orchards was 131.54cm, whereas in G. nobilis negative orchards mean tree girth was 129.03cm. A difference of only 2.51cm. Tree girth range was 30cm larger in G. nobilis negative orchards than G. nobilis positive orchards. |
| Tree Girth - Statistical Analysis - Tree Level | None | H0 was accepted for all tree species - There was no difference in median girth between specific fruit trees with G. nobilis present and specific fruit trees with G. nobilis absent. |
| Tree Girth - Statistical Analysis - Orchard Level | Significant difference (at the 95% confidence level) in median girth of P. avium trees between orchards which had G. nobilis present, and those where it was absent. No difference between girth of G. nobilis positive and negative orchards with any other tree species. | P-value for P. avium was 0.028, therefore the H1 hypothesis was accepted, that there was a difference in median girth of P. avium trees between orchards with G. nobilis present and those where it was absent. |
| Adjacent Orchard Surrounding Habitat | G. nobilis seemed to prefer a higher diversity of surrounding habitat, with slightly higher proportion of orchards. | Surrounding habitat of G. nobilis positive orchards was composed of 18% orchards, in G. nobilis negative orchards, this was 12%. There were 6 different categories of surrounding habitat in G. nobilis positive orchards, compared with 4 categories in G. nobilis negative orchards. |
| Orchard Management | Less intense management and therefore a higher amount of dead wood could be preferred by G. nobilis | Less intense management techniques were more prominent in G. nobilis positive orchards. In orchards where dead wood was left and no management combined had the highest percentage of 38% in G. nobilis positive orchards and 19% in G. nobilis negative orchards. In G. nobilis negative orchards, there were no orchards where dead wood was intentionally left in situ. |
| Orchard Microclimate: | ||
| Tree Density | None | Statistical analysis showed that there was no difference in orchard tree density and G. nobilis presence (H0 accepted). However, 75% of G. nobilis negative orchards did have tree densities of 94 trees per hectare and above. |
| Orchard Size | None | H0 was accepted, there was no difference in orchard size between G. nobilis positive orchards and G. nobilis negative orchards |
| Orchard Aspect | None | 41.6% of G. nobilis positive orchards were situated on a South facing slope. 75% of G. nobilis negative orchards were on non South facing slopes. |
Table 1. Summary of the results of the study.
Discussion
On the tree level, P. domestica trees had the highest percentage of G. nobilis presence (33.33%), with P. avium second (22.58%). However, on the orchard level, P. domestica was the least preferred (33.33%) and P. avium was most favoured (83.87%). This could suggest that G. nobilis preferably use individual trees of this species to inhabit rather than just orchards with this tree in. However, P. domestica had to be eliminated from statistical tests as there was a low abundance of the species in this study. The results in this report suggest that P. avium trees are also preferred over other species (except P. domestica) for colonisation, as G. nobilis are commonly found in purely P. avium orchards; in orchards where P. avium and other species are present; and G. nobilis colonised a high number of P. avium trees (21 Trees, 22.58% of all G. nobilis positive trees).
Heartwood decay fungi such as Sulphur Polypore Laetiporus sulphurous are a common feature of old traditional orchards (Natural England, 2010). These fungi do not harm the host tree, but attack the cellulose of the tree cells resulting in red rot and white rot, creating cavities in the tree, which G. nobilis and other wildlife can exploit (Natural England, 2010). The fungi soften the wood making it accessible for saproxylic invertebrates to feed on (Woodland Trust, 2011). G. nobilis preferentially eat away at the wood mould inside the tree column, which has been attacked by L. sulphurous (Woodland Trust, 2011). L. sulphurous is a particularly common on P. domestica and P. avium trees (Smith, 1988). This could suggest why P. domestica and P. avium trees were favoured by G. nobilis in this report. Because of these fungi, trees have softer wood mould contained within them, which is easier for G. nobilis to digest.
A higher diversity of tree species in each individual orchard was possibly preferred by G. nobilis, which has not been shown in previous studies.
In G. nobilis positive orchards and trees the tree girth of all species, accounted together, had a larger girth. In G. nobilis negative orchards and trees the range of tree girths was smaller. This suggests there was a small range of large tree girths where the dead wood was suitable for G. nobilis. PTES (2010) have stated that G. nobilis inhabits orchard fruit trees with a girth of between 62cm and 116cm, a range of 54cm. However, in this study, G. nobilis was found in trees between 55cm and 210cm; a much larger range of 155cm. This could be due to different tree species producing wood mould at different ages (Dubois et al. 2009) who stated that saproxylic beetles preferred or avoided species according to the amount of wood mould found in the tree, this being due to the characteristics of the tree itself.
Tree girth of all tree species were different in G. nobilis positive trees compared to G. nobilis negative trees: mean 138.29cm in G. nobilis positive trees and 127.02cm in G. nobilis negative trees. This could equate to about ten years age difference, which could affect how much dead wood is in the tree (PTES, 2010). The larger trees could therefore be older, and produce more wood mould (Ranius, 2002) which G. nobilis larvae could utilise as a food source.
P. avium trees had a significant difference in tree girth between the orchards that did and did not contain G. nobilis (p-value 0.028 at 95% confidence level). G. nobilis positive orchards contained P. avium trees 25.61cm larger on average than in G. nobilis negative orchards. This could be due to the age of the P. avium trees; older trees could contain more suitable dead wood conditions for G. nobilis (Dubois et al., 2009). In Ranius’ (2002) study, several saproxylic species occurred with higher frequencies in trees with a larger girth, this being due to larger amounts of wood mould found.
These results could, however, simply suggest colonisation at different times. The older trees could have sustained G. nobilis populations for a much longer time than populations in the younger trees (Ranius, 2002). The larger the tree girth, which signifies older trees, the longer species can persist because of the larger amounts of wood mould present (Ranius, 2002).
The small girths at The Cherries Orchard consisted of four different species of tree; P. domestica (55cm GBH), Malus domestica (80cm GBH), P. communis (65cm GBH) and P. avium (95cm GBH). Therefore this suggests that it is age and not tree species that G. nobilis prefers (Alexander, 2002). However, the P. communis tree with a GBH of 65cm had a rot hole, created by a woodpecker. (GBH = Girth at Breast Height). This rot hole, where woodpeckers have acted as allogenic engineers, may not have been caused by the natural decay process of a tree, but it could also contain G. nobilis (Jones et al., 1994). The softest part of a tree is usually chosen by woodpeckers, though the outer layer must be strong enough to support the excavated cavity (Jones et al. 1994) and in this case the softest part of the tree is the rotten core. This could suggest that the harder outside of the tree had not started decaying, and therefore the decay inside the tree was not exposed for G. nobilis colonisation but as the woodpecker pecked through the harder tree exterior, the wood mould inside became accessible for G. nobilis to inhabit (PTES, 2010). The M. domestica (GBH 80cm) and the P. avium (GBH 95cm) at The Cherries were both dead. Therefore, dead wood was available as the tree was being broken down by organisms in the natural nutrient recycling process of decomposition, so producing soft dead wood that G. nobilis can digest (Whitehead 2003; Blakesley and Buckley 2010). These trees could have died at a young age, hence their relatively small girths, but as they have died, they can provide the dead wood that G. nobilis favours (PTES, 2010).
It is suggested that small clusters of G. nobilis positive orchards interact at some levels. This was shown in the results from the surrounding orchard habitat. In G. nobilis positive orchards, 41% of the surrounding habitat was fields and 18% was orchards. The high proportion of fields could have been old orchards which have been cleared for agriculture. The proportion of orchard surrounding habitat around the G. nobilis positive orchards in this report could suggest that G. nobilis interact with these surrounding orchards on the metapopulation scale for food and mates. These metapopulations could be areas that G. nobilis has been forced to occupy, since their first choice of habitat locations have been altered (Keble 2001). The fields present unsuitable habitat for G. nobilis, whilst the orchards are the suitable habitat within which they interact. 18% of the orchard surrounding habitat is woodland, which could mean that G. nobilis interacts with the ancient woodland of the Wyre Forest. There are two suggestions as to why this might be. Franc et al. (2007) suggested that saproxylic beetles interact with woodland as it provides a corridor of dead wood for colonisation. A higher number of different surrounding habitats occur in G. nobilis positive orchards. As Økland et al. (1996) observed a richer mosaic of different habitats increased saproxylic species presence, due to the different habitats provided, creating the specific niches for saproxylic species.
In G. nobilis negative orchards there was a higher proportion of woodland surrounding habitat (25%) compared to G. nobilis positive orchards (18%). Denser woodland may not facilitate the movement of adults (Kindlmann, 2005) and a large canopy provides a habitat which is too cold and damp for G. nobilis (Alexander, 2008). For example at Lodge Hill Farm a G. nobilis negative orchard is surrounded by woodland on three sides. It could be a G. nobilis negative orchard because colonisation of this orchard could be difficult, as G. nobilis may not be able to penetrate through the dense woodland to find this orchard site (Franc et al. 2007). Lodge Hill Farm was not in close enough proximity (within 700m) to any other G. nobilis positive orchards for possible colonisation.
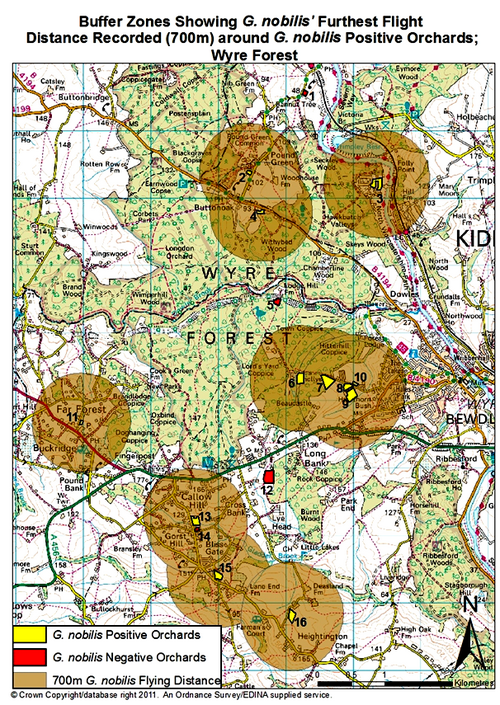
As Whitehead (2003) identified, the largest distance that G. nobilis has been recorded to fly is 700m. Fig. 1. shows G. nobilis positive sites, and a 700m buffer zone around each one. This shows that there could possibly be two metapopulations in the area, one around The Cottage and Dreamskerry (2 and 4), and another around Bowcastle Farm (7, 8 and 9), Hole Farm (10) and Uncllys (7). These two areas had G. nobilis populations within 700m of each other, therefore they could potentially interact at some level, for food sources, trees to colonise and mates (Levins, 1969).
However, other G. nobilis positive orchards such as Far Forest School (11), The Latchetts (16), and Hawkbatch Farm (3) are not in close proximity to any other G. nobilis positive orchards. It could be that these orchards were once connected by other orchards, but have suffered from habitat fragmentation. As Whitehead (2006) identified, these isolated G. nobilis positive orchards could prove that beetles and larvae can survive in one tree for several generations. These G. nobilis populations could have inhabited the trees in these orchards utilising the wood mould resource for many years (Whitehead, 2007). As the larvae develop, their mandibles become larger and stronger, enabling the larvae to eat their way through the tree trunk where the wood may be a lot harder and where there is less water and oxygen (Whitehead, 2006).
It can be seen that three out of four G. nobilis negative orchards are not within a 700m flight distance of any G. nobilis positive orchards. This would suggest that it is hard for G. nobilis to colonise these negative orchards as they are not in close proximity to any G. nobilis populations. However, Brooklands was in very close proximity to a G. nobilis positive site: The Cherries. Brooklands was managed as a garden, so there was no dead wood present, suggesting why G. nobilis had not been found inhabiting this orchard (PTES, 2010). In all G. nobilis negative orchards, dead wood had been removed by management practices such as pruning. In G. nobilis positive orchards, 27% of orchards had the dead wood left intentionally, and 11% of G. nobilis positive orchards had no management, which both meant that a considerable amount of dead wood was present. This would suggest that the higher the proportion of dead wood, the greater the chances are of species like G. nobilis inhabiting these orchards, as a dead wood resource is crucial to their life cycle (Speight 1989; Alexander 2008; PTES 2010; Whitehead 2003). In 1% of G. nobilis positive orchards, orchard trees had been pollarded, and in 11% of G. nobilis positive orchards, pruning was apparent. Pollarding and pruning can create crevices and rot holes as the cuts created by a saw mimic natural tears providing colonisation sites for saproxylic species (Fay and Berker 1997; Forestry Commission 2002). Therefore, 50% of G. nobilis positive orchards in the Wyre Forest area involved management practices that encouraged dead wood to form on living trees, or kept dead wood in situ in the orchards, without clearance.
The UK BAP (2010) stated that one of the threats to G. nobilis is the loss of nectar and pollen sources through inappropriate management of orchard grassland, such as intense grazing. In G. nobilis negative orchards, the largest proportion of management was grazing (39%), which could be why G. nobilis are not found in these orchards as a food source is not available. The grazing animals could have eaten the plants such as Hogweed Heracleum sphondylium and Yarrow Achillea millefolium before they come into flower so the nectar that G. nobilis feed on was not available.
Even though it did not show up on statistical tests (p-value 0.5852 at 95% confidence level), a majority of G. nobilis positive orchards had the lowest tree densities. This is probably due to the warm microclimate that a low tree density creates. Each individual tree is more sun exposed as there is less shading (Ranius, 2002). Beetles in tree hollows prefer a stable warm temperature and lack of moisture (Kelner-Pillault 1974), which is what a south facing slope provides. It could also mean that the umbel flowers which G. nobilis commonly feeds on have enough light to grow on the orchard floor (Database of Insects and their Food Plants, 2010), as they are species that need plentiful amounts of light to grow (Heywood, 1971).
Orchard size did not affect whether or not G. nobilis was present (p-value 0.2029 at 95% confidence level). This suggests that it is the characteristics of the habitat inside the orchard, rather than the size of the orchard, which could attract or deter G. nobilis.
In relation to orchard aspect, there was not a strong relationship between G. nobilis presence and South facing slopes. 75% of G. nobilis negative orchards were on not on south facing slopes, which is as expected due to the colder microclimate. Out of all G. nobilis positive sites, 41.6% were on south facing slopes, a far lower percentage than predicted. Ranius and Jansson (2000) found that saproxylic beetles preferred sun exposed trees, but the relationship was not found in Ranius’ (2002) survey. Sun exposure may have changed in the study sites during the last few years, as a majority of the trees now had shading due to dense surrounding vegetation (Ranuis, 2002). However, the saproxylic invertebrates still inhabited the trees because of favoured conditions in the past. The only G. nobilis positive orchard in this survey with dense surrounding vegetation was The Cottage but this was sun exposed on a South facing slope.
On closer inspection, looking at microclimate as a whole, there does seem to be a relationship. Apart from Far Forest School, all non South facing slopes had the lowest tree densities. The Cherries had the highest tree density, but as this was on a South facing slope. Tree density did not seem to deter G. nobilis. G. nobilis positive orchards either need to be south facing or have a low tree density, because G. nobilis being cold blooded, need heat (Ball 1985).
At this stage in the research, there were only some habitat parameter patterns that emerged. The current literature suggests patterns of habitat parameters can be explained. There are possible anomalies and limitations to this report, such as Tarn, which pose further questions for their findings. At the beginning of this study sixteen orchards were identified: six orchards with recorded G. nobilis populations and ten without. When undertaking this research new populations were recorded in a higher number of orchards than previously expected. This will contribute to the Worcestershire Biological Records Centre’s records of the species in this area. However, a previously recorded G. nobilis positive orchard came out as G. nobilis negative in this study: Tarn. This is most likely to be due to the random methods of sampling in this report. This highlights the need for more research into G. nobilis in the Wyre Forest area.
However, it needs to be noted that although habitat parameter relationships have emerged with relation to G. nobilis presence, and there is literature to support these explanations, no real conclusions can be made, due to a data set with only four G. nobilis negative orchards. It is hoped that future research can use the data in this report as a baseline for further studies on G. nobilis in the Wyre Forest area.
As this study relied on the presence or absence of G. nobilis frass which may persist in the wood mould over several years, the occupancy patterns observed may not reflect recent habitat changes, but may be to a result from the situation in the past (Ranius 2002).
References
Alexander, K. 2004. The Noble Chafer Gnorimus nobilis in Gloucestershire- a Report on the 2003 Survey, Unpublished Report for the People’s Trust for Endangered Species.
Alexander, K. 2005. The Noble Chafer Gnorimus nobilis in Gloucestershire- a Report on the 2004 Survey, Unpublished Report for the People’s Trust for Endangered Species.
Alexander, K. 2008. The special importance of traditional orchards for Invertebrate conservation, with a case study of the BAP species the Noble Chafer Gnorimus nobilis, in Rotherham, I. 2008. Orchards and Groves: Their History, Ecology, Culture and Archaeology, Landscape and Ecology, 7.
Alexander, K. 2010. Saproxylic Beetles, In: Newton, A. 2010. Biodiversity in the New Forest, Information Press, Oxford.
Allen, A. 1960. The history and present day status of Gnorimus variabilis L. (Col., Scarabaeidae) in Britain, Entomologist’s Record and Journal of Variation, 72: 129-132.
Baille, J., Hilton-Taylor, C., and Stuart, S. 2004. 2004 IUCN Red List of Threatened Species: A Global Assessment. International Union for Conservation of Nature, Cambridge, UK.
Ball, G.1985. Taxonomy, Phylogeny and Zoogeography of Beetles and Ants, Dr W. Junk Publishers, Dordrecht
Berkshire Biodiversity Action Plan. 2010) Traditional Orchards, [online] Available at: http://www.berksbap.org/traditional-orchards-1 [Accessed 20.08.2010]
Blakesley, D., and Buckley, G. 2010. Managing Your Woodland for Wildlife, Pisces Publications, Newbury
Boatman, N., Crocker, D., Hart, A., Roelofs, W., Smith, R., Holland, J., Lutman, P., Brown, V., and Ramsay, A. 2004. Development of a scheme for the assessment of risks to wider biodiversity arising from the use of pesticides, Final report to DEFRA
Bouget, C., Brustel, H., and Zagatti, P. 2008. The French information system on saproxylic beetle ecology (FRISBEE): An ecological and taxonomical database to help with the assessment of forest conservation status, Revue d' Ecologie (la Terre et la Vie) 10:33-36.
Buse, J., Schröder, B., and Assmann, T. 2007. Modelling habitat and spatial distribution of an endangered longhorn beetle- a case study for saproxylic insect conservation, Biological Conservation, 137: 372-381.
Callaway, R., Reinhart, K., Moore, G., Moore D., and Pennings, S. 2002. Epiphyte host preferences and host traits: mechanisms for species-specific interactions, Population Ecology, 132(2):221–230.
Database of Insects and their Food Plants, (2010), Coleoptera >> Scarabaeidae >> Gnorimus nobilis (L.) [online], Available at: http://www.brc.ac.uk/DBIF/invertebratesresults.aspx?insectid=4100, [Accessed 16.10.2010].
Davies, K., Margules, C., and Lawrence, J. 2000. Which traits of species predict population declines in experimental forest fragments?, Ecology, 81: 1450–1461.
Dubois, G., Vignon, V., Delettre, Y., Rantier, Y., Vernon, P., and Burel, F. 2009. Factors affecting the occurrence of the endangered saproxylic beetle Osmoderma eremite (Scopoli, 1763) (Coleoptera: Cetoniidae) in an agricultural landscape, Landscape Planning 91:152-159.
Eccleson, P. 2007. Noble Chafer found in Kent [online], Available at: http://www.telegraph.co.uk/earth/earthnews/3317524/Noble-chafer-beetle-found-in-Kent.html [Accessed 23.09.2010].
Ehnström, B. 2001. Leaving dead wood for insects in boreal forests - suggestions for the future, Scandinavian Journal of Forest Research 3: 91–98.
European Red List. 2010. An Introduction to Saproxylic Beetles [online], Available at: http://ec.europa.eu/environment/nature/conservation/species/redlist/beetles/introduction.htm, [Accessed 08.01.2011].
Fay, N., & Berker, N. 1997 Veteran Trees Initiative: Specialist Survey Method, English Nature.
Ferris-Kaan, R., Londsdale, D., and Winter, T. 1993. The Conservation Management of Dead Wood in Forests, In: Research Information Note 241, Forestry Authority, Holt Alice.
Forestry Commission. 2010. Wyre Forest Orchard Restoration Underway [online], Available at: http://www.forestry.gov.uk/newsrele.nsf/WebPressReleases/E2CCBE6B9123CE108025754B0053A3AC [Accessed 1.10.2010].
Forestry Commission. 2002. Life in the Dead Wood, A Guide to managing deadwood in Forestry Commission Forests, Forest Enterprise, Bristol.
Franc, N., Götmark, F., Økland, B., Nordén, H., and Paltto, H. 2007. Factors and Scales Potentially Important for Saproxylic Beetles in Temperate Mixed Oak Forest, Biological Conservation, 136:86-98
Fry, R., and Lonsdale, D., (1991), Habitat Conservation for Insects - A Neglected Green Issue, The Amateur Entomologists’ Society, London.
Google Maps, (2011), Google Maps [online], Available at: http://maps.google.co.uk/ [Accessed 05.01.2011].
Green, H. 2004. The Noble Chafer Gnorimus nobilis: records needed, Worcestershire Record, 16:10-11 [online], http://www.wbrc.org.uk/WorcRecd/Issue%2016/noble_chafer.htm [Accessed 10.08.2010]
Green, P., and Peterken, G. 1997. Variation in the Amount of Dead Wood in the Woodlands of the Lower Wye Valley, UK in Relation to the Intensity of Management, Forestry Ecology and Management, 98:229–238.
Groombridge, B. 1992 Global biodiversity : Status of the Earth's Living Resources - World Conservation Monitoring Centre, Chapman & Hall, London.
Grove, S. 2002. Saproxylic Insect Ecology and the Sustainable Management of Forests, Annual Review of Ecology, Evolution and Systematics, 33:1–23.
Hampshire Biodiversity Partnership. 2001. Noble Chafer, Gnorimus nobilis: Species Action Plan [online], Available at: http://www.hampshirebiodiversity.org.uk/pdf/PublishedPlans/NobleChaferVerIISAP.pdf, [Accessed 10.10.10].
Harding, P., and Rose F. 1986. Pasture-woodlands in Lowland Britain; A Review of Their Importance for Wildlife Conservation, Institute of Terrestrial Ecology, Huntingdon, UK.
Heywood, V. 1971. The Biology and Chemistry of the Umbelliferae: Linnean Society of London Journal of Botany, Academic Press, New York.
Hickin, N. 1978 The Natural History of an English Forest; The Wild Life of Wyre, Westmid Supplies, Shrewsbury.
Holland, P., and Steyn, D. 1975. Vegetation responses to latitudinal variations in slope angle and aspect, Journal of Biogeography, 2:179-183.
Hulme, M., Turnpenny, J., and Jenkins, G. 2002. Climate Change Scenarios for the United Kingdom: the UKCIP02 Briefing Report, Tyndall Centre for Climate Change Research, Norwich, UK.
IUCN. 2010. The IUCN Red List of Threatened Species [online], Available at: http://www.iucnredlist.org/ [Accessed 10.08.2010].
Jones, C., Lawton, J., and Shachak, M. 1994. Organisms as ecosystem engineers, Okios, 69:373-386.
Jonsson, B., and Kruys, N. 2001. Ecology of Woody Debris in Boreal Forests, Ecological Bulletins, 49:1–283.
Keble, D. 2001. Wild Things; Noble Chafer Beetle, [online] Available at: http://www.telegraph.co.uk/gardening/3292356/Wild-things-noble-chafer-beetle.html [Accessed 25.08.2010].
Keech, D. 2000. Common Ground Book of Orchards: Community, Conservation and Culture, Common Ground, London.
Kelner-Pillault, S. 1974. Ecological Study of the Stand Entomology Terraux Hollow Tree (Chestnuts and Willows), Bulletin d’ Ecologie, 5:123–156.
Key, R. 1992. What are Saproxylic Invertebrates? In: Kirby, K., & Drake C. 1992). Dead Wood Matters: The Ecology and Conservation of Saproxylic Invertebrates in Britain. 1992, English Nature, English Nature Science: 7.
Key, R. 2011. Noble Chafer (Gnorimus nobilis) [online], Available at: http://www.arkive.org/noble-chafer/gnorimus-nobilis/image-A6264.html#text=All [Accessed 24.01.2011].
Kindlmann, P., Aviron, S., and Burel, F. 2005 When is landscape matrix important for determining animal fluxes between resource patches? Ecological Complex, 2:150–158
Kirby, K. 1992. Accumulation of dead wood: a missing ingredient in coppicing?, in G P Buckley [editor], Ecology and Management of Coppice Woodlands, Chapman and Hall, London.
Kouki, J., Löfman, S., Martikainen, P., Rouvinen, S., and Uotila, A. 2001. Forest fragmentation in Fennoscandia: linking habitat requirements of wood-associated threatened species to landscape and habitat changes, Scandinavian Journal of Forest Research, 3:27–37.
Krejčík, S. 2008. Gnorimus nobilis Linnaeus [online], Available at: http://www.eol.org/pages/141887, [Accessed 01.12.2010].
Levins, R. 1969. Some demographic and genetic consequences of environmental heterogeneity for biological control, Bulletin of the Entomological Society of America, 15:237-240.
Martikainen, P., Siitonen, J., Kaila, L., Punttila, P., and Rauh, J. 1999. Bark beetles (Coleoptera, Scolytidae) and associated beetle species in mature managed and old-growth boreal forests in southern Finland, Forest Ecology and Management, 116:233–245.
Marrotte, E. 2003. Why Fruit Trees Fail to Bear [online], Available at: http://www.uri.edu/ce/factsheets/sheets/fruittreesfail.html [Accessed 21.03.2011].
National Trust. 2010. Apples and Orchards, [online] Available at: http://www.nationaltrust.org.uk/main/w-chl/w-countryside_environment/w-nature/w-nature-orchard-restoration/w-nature-orchards-history.htm [Accessed 10.08.2010].
Natural England. 2010. Traditional Orchards: Orchards and Wildlife [online], Available at: http://naturalengland.etraderstores.com/NaturalEnglandShop/TIN012 [Accessed 23.03.2011].
Økland, B., Bakke, A., Hagvar, S., and Kvamme, T. 1996. What factors influence the diversity of saproxylic beetles? A multi-scaled study from a spruce forest in southern Norway, Biodiversity and Conservation, 5:75–100.
People’s Trust for Endangered Species 2010. The Noble Chafer beetle [online] Available at: http://www.ptes.org/?page=170 [Accessed 08.08.2010].
Prime, C. and Deacock, R. 1970. Trees and shrubs and their identification in summer or winter, Heffer, Cambridge
Rackham, O. 2001. Trees and Woodland in the British Landscape: The Complete History of Britain's Trees, Woods and Hedgerows, Phoenix Press, London.
Ranuis, T. 2002. Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden, Biological Conservation, 103 (1):85-91.
Ranius, T., and Jansson, N. 2000. The influence of forest re-growth, original canopy cover and tree size on saproxylic beetles associated with old oaks, Biological Conservation, 95:85–94.
Ranius, T., and Jansson, N. 2002. A comparison of three methods to survey saproxylic beetles in hollow oaks, Biodiversity and Conservation, 11:1759-1771.
Ranney, J., Bruner, M., and Levenson, J. 1981. The Importance of Edge in the Structure and Dynamics of Forest Islands, In Burgess, R. & Sharpe, D. Forest island dynamics in man-dominated landscapes, Springer, New York.
Read, H. 2000. Veteran Trees: A Guide to Good Management, Natural England.
Rosenberg, B. 1983. Microclimate: The Biological Environment, John Wiley and Sons, London.
Saint-Germain, M., Drapeau, P., and Buddle, C. 2007. Host-use patterns of saproxylic phloeophagous and xylophagous coleoptera adults and larvae along the decay gradient in standing dead Black Spruce and Aspen, Ecography, 30:737-748.
Saunders, D., Hobbs, R., and Margules, C. 1991. Biological Consequences of Ecosystem Fragmentation: A Review, Conservation Biology, 5:18–32.
Similä, M., Kouki, J., and Martikainen, P. 2003. Saproxylic beetles in managed and semi-natural Scots Pine forests: quality of dead wood matters, Forest Ecology and Management, 174:365–381.
Smart, M., and Winnall, R. 2006. The Biodiversity of Tree Traditional Orchards within the Wyre Forest SSSI in Worcestershire: A Survey by the Wyre Forest Study Group, English Nature Research Report 707: 1-89.
Smith, I. 1988. European Handbook of Plant Diseases, Blackwell Publishing, Oxford.
Smith, M.(2004. Just Leave the Dead to Rot, The Guardian newspaper, March 25th 2004, London.
Smith, M. 2005. Matt Smith’s Report 2005; Worcestershire Noble Chafer Survey, Unpublished Report to People’s Trust for Endangered Species
Smith, M. 2003 Saproxylic beetles in Britain, an overview of the status and distribution of four biodiversity action plan species, In Bowen, C., Proceedings of the second pan-European conference on Saproxylic Beetles, PTES, London.
Smith, R., and Smith, T. 2002. Elements of Ecology, 5th Edition, Benjamin Cummings Publishing, New York.
Speight, M. 1989. Saproxylic Invertebrates and their Conservation, Council of Europe, Strasbourg.
UK Biodiversity Action Plan. 2010. Species Action Plan; The Noble Chafer Gnorimus nobilis [online] Available at: http://www.ukbap.org.uk/UKPlans.aspx?ID=326#1 [Accessed 12.10.2010].
UK Biodiversity Steering Group. 1999. Action Plans: Volume IV-Invertebrates, English Nature, Peterborough, England.
Väisänen, R., Biström, O., Heliövaara, K. 1993. Sub-cortical Coleoptera in dead pines and spruces: is primeval species composition maintained in managed forests? Biodiversity and Conservation, 2:95–113.
Vernon, P., and Vannier, G. 2001. Freezing susceptibility and freezing tolerance in Palaearctic Cetoniidae (Coleoptera), Canadian Journal of Zoology, 79:67–74.
Wermelinger, B., Flückiger, P., Obrist, M., and Duelli, P. 2007. Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycide, Scolytine) across sections of forest edges, Journal of Applied Entomology, 131(2):104-114.
Wheater, C., and Cook, P. 2003. Using Statistics to Understand the Environment, Routledge, London.
Whitehead, P. 1990. Further Observations on Gnorimus nobilis (L.) (Col., Scarabaeidae) in Worcestershire. Entomologist’s Monthly Magazine, 126:110.
Whitehead, P. 1990a. Rare Beetles from an apple tree in Worcestershire, Entomologist’s Monthly Magazine, 126:236.
Whitehead, P. 1997. Invertebrates of pome and stone fruit orchards in the English Wyre Forest, July 1997, with particular regard to Gnorimus nobilis (L., 1758) (Col., Scarabaeidae) pp. 1-8, Unpublished Report for English Nature.
Whitehead, P. 1999. The 1999 Worcestershire Survey for Gnorimus nobilis (L., 1758) (Col., Scarabaeidae), Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2000. The 2000 Gloucestershire survey for Gnorimus nobilis (L., 1758) (Col., Scarabaeidae), Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2000a. The 2000 Worcestershire Survey for Gnorimus nobilis (L., 1758) (Col., Scarabaeidae), Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2001. The 2001 Gloucestershire Survey for Aleurosticus nobilis (L., 1758) (Col., Scarabaeidae), Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2001a. The 2001 Worcestershire Survey for Aleurosticus nobilis (L., 1758) (Col., Scarabaeidae), Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2001b. Evidence of the Noble Chafer Aleurosticus nobilis (L., 1758) (Col., Scarabaeidae) in Warwickshire during 2001, Unpublished Report for the People’s Trust for Endangered Species.
Whitehead, P. 2003. The Noble Chafer Aleurostictus nobilis (L., 1758) (Col., Scarabaeidae) in Britain, Proceedings of the Second pan-European conference on Saproxylic Beetles; People’s Trust for Endangered Species, London.
Whitehead, P. 2006. Larvae of Noble Chafer Aleurostictus nobilis (L.) (Col., Scarabaeidae) developing terrestrially in Gloucestershire, Entomologist's Monthly Magazine, 142:228.
Whitehead, P. 2007. Noble Chafer Aleurostictus nobilis (L., 1758) (Col., Scarabaeidae) Breeding externally on a pear tree, Entomologist's Monthly Magazine, 143:206.
Woodland Trust. 2011. Ancient Trees and Wildlife [online], Available at: http://frontpage.woodland-trust.org.uk/ancient-tree-forum/information/ecology/natural_info.htm#Fungi and trees [Accessed 25.03.2011].
Worcestershire Biodiversity Partnership. 2008. Traditional Orchards Habitat Action Plan, H2 Traditional Orchard BAP, Unpublished Report.
WWF. 2004. Deadwood- Living forests, WWF Report October 2004.
Image
Fig. 1. Map of the Gnorimus nobilis positive and negative sites in this study. The buffer zones represent 700m around G. nobilis positive orchards; the largest recorded distance of G. nobilis flight (Whitehead 2003).